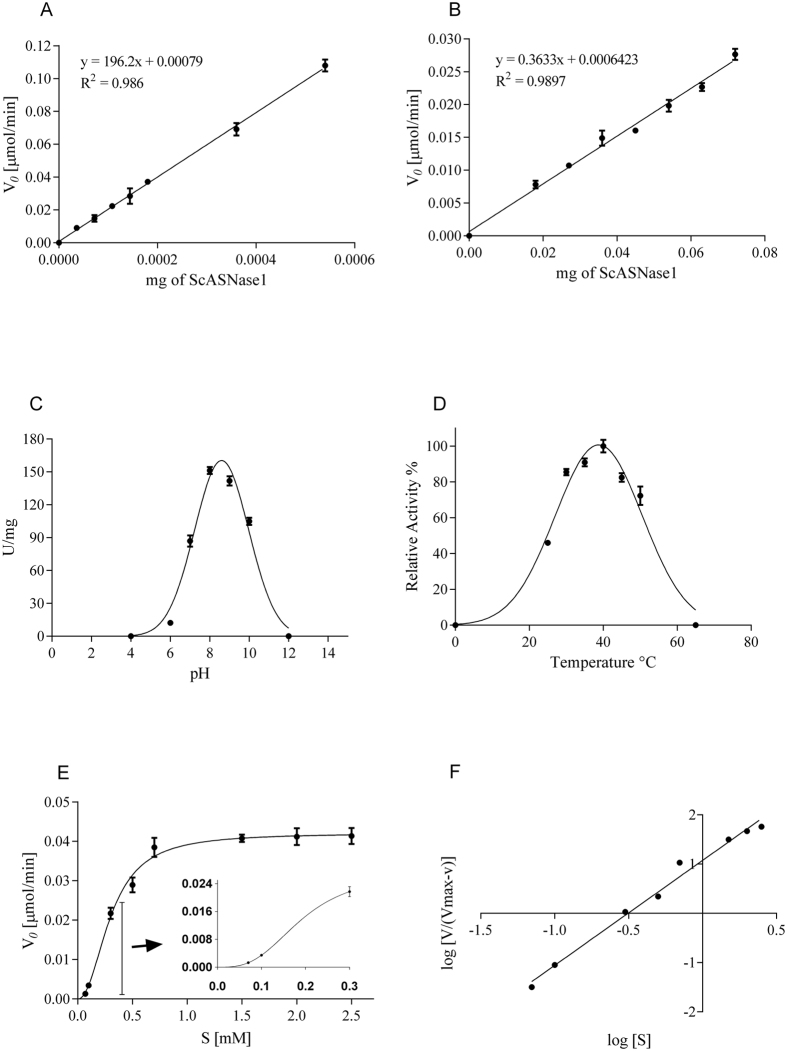
Figure 1.
Determination of specific activity, optimum reaction conditions and kinetic characterisation for ScASNase1 enzyme. (A) Specific activity of ScASNase1 to hydrolyse L-Asn: Plot of the reaction velocities (V0) of L-Asn hydrolysis as a function of mg of purified ScASNase1 as measured by Nessler’s reagent. (B) Specific activity of ScASNase1 for L-Gln as measured by coupled assay with NADH oxidation: Plot of the reaction velocities (V0) of L-Gln hydrolysis as a function of mg of purified ScASNase1. (C) The effect of pH was determined in different buffers (acetate pH 4.0; sodium phosphate pH 6.0, 7.0 and 12.0; Tris–HCl pH 8.0 and 9.0; sodium bicarbonate pH 10.0). (D) Optimum temperature was determined by measuring the specific activity in the range from 25 °C to 50 °C. (E) ScASNase1 kinetics, activity dependence on a substrate concentration plot. The inset shows the sigmoidal profile of the enzyme at lower substrate concentrations. (F) Hill plot of the data. Points in the graph represent means ± SD (n = 3).