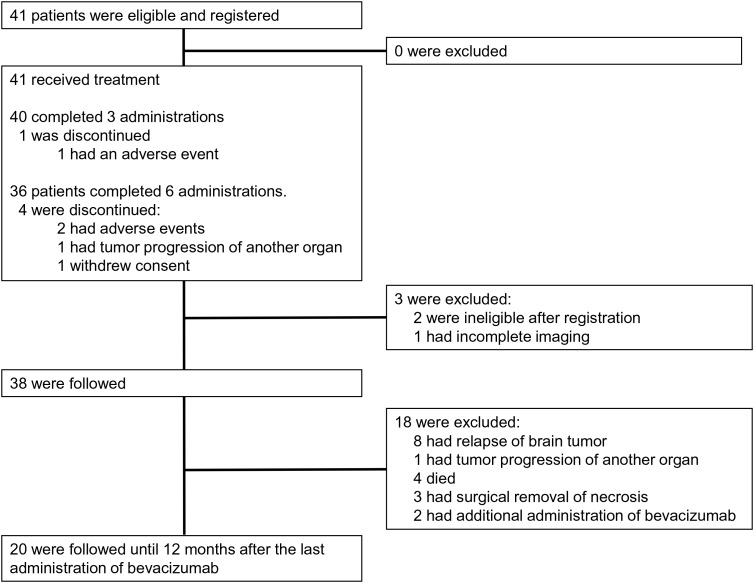
Fig. 1.
CONSORT diagram. Of the 41 patients enrolled in this trial, 40 underwent 3 cycles of bevacizumab. One patient had an adverse event (AE) leading to the discontinuation of bevacizumab. Thirty-eight patients were followed up as the full analysis set (FAS). Thirty-six patients underwent 6 administrations of bevacizumab. Of the 4 patients who discontinued bevacizumab, 2 had AEs. Primary organ tumor recurred in 1 patient and consent was withdrawn by 1 patient. Thirty-eight patients were followed, and 20 patients were completely followed until 12 months after the last administration of bevacizumab (the per protocol set [PPS]).