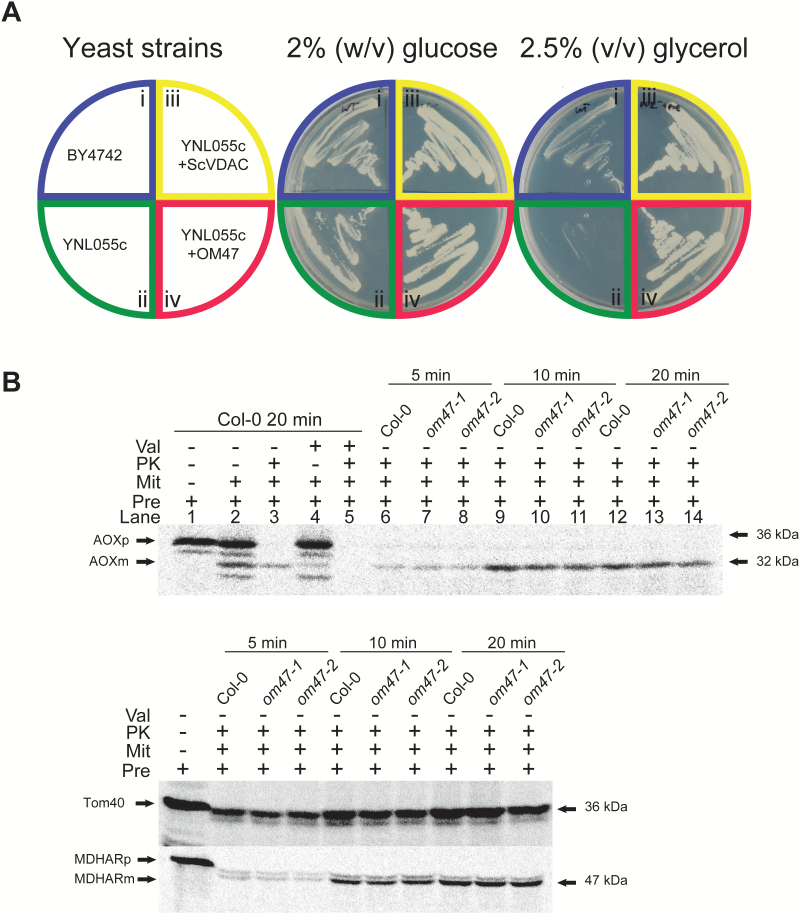
Fig. 2.
Arabidopsis thaliana OM47 can functionally replace yeast VDAC. (A) Complementation assay of the yeast (Saccharomyces cerevisiae) ScVDAC protein with OM47. The yeast VDAC deletion strain ΔYNL055c was transformed with the OM47 and ScVDAC. The ΔYNL055c+OM47 complemented strain grew on the medium with glycerol, suggesting that OM47 can functionally replace the ScVDAC protein. ΔYNL055c+ScVDAC was used as a positive control. (B) In vitro import assays of precursor proteins into isolated mitochondria from 2-week-old wild-type and om47 plants. Upper panel: to verify the import assay, the precursor for alternative oxidase (AOXp) was imported into mitochondria isolated from wild-type plants, in the presence and absence of valinomycin, that dissipates the membrane potential across the inner membrane required for protein import into or across the inner membrane. A protease-protected product (mature AOX, AOXm) was detected (lane 3) that was not present in the presence of valinomycin (lane 5). No difference in the amount of protein import between mitochondria isolated from wild-type plants (Col-0) and om47 mutants could be detected over 20min. Lower panel: the TOM40 and monohydroascorbate reductase (MDAR) precursor proteins were used instead of AOX precursor as in lanes 1 and 6–14 in the upper panel. Val, valinomycin; PK, protease K; Mit, mitochondria, Pre, precursor protein.