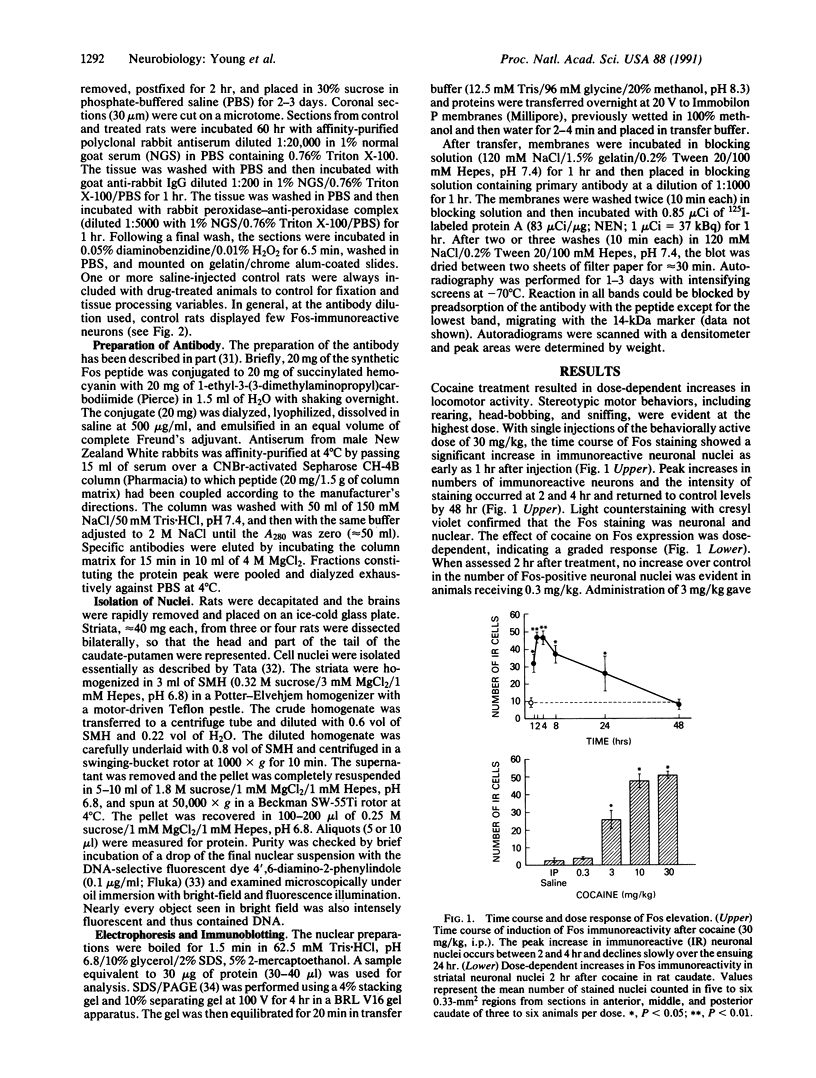
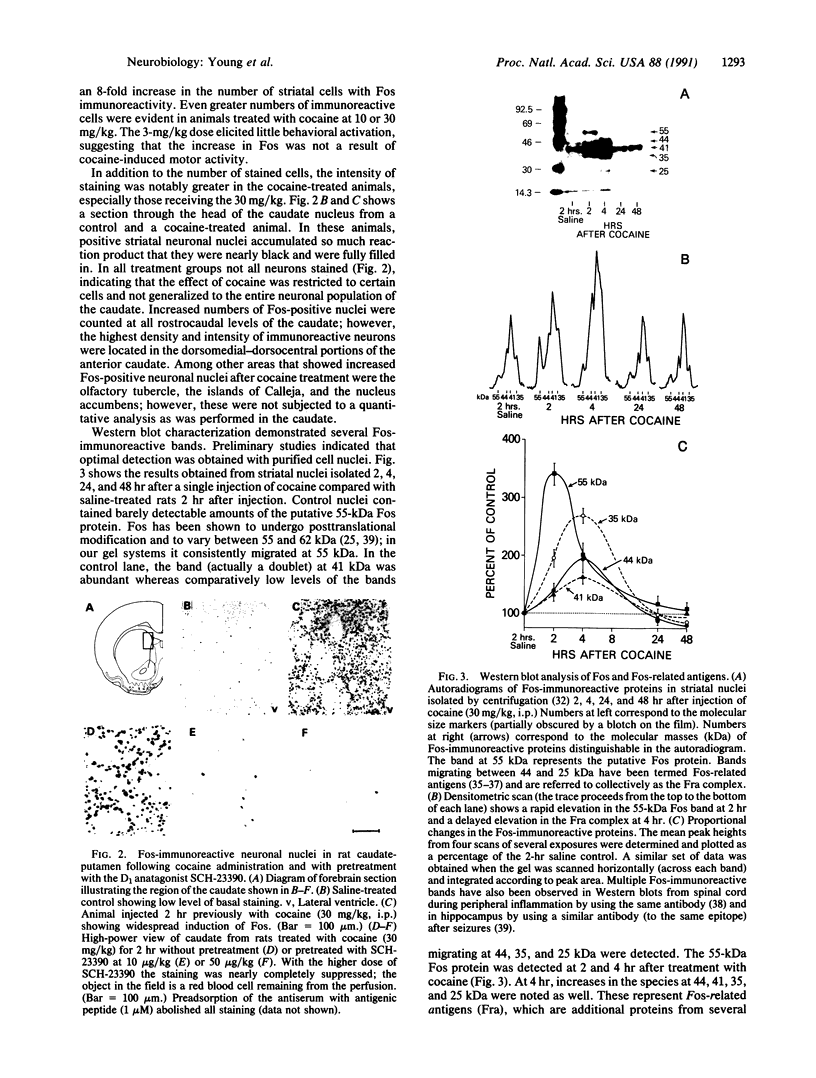
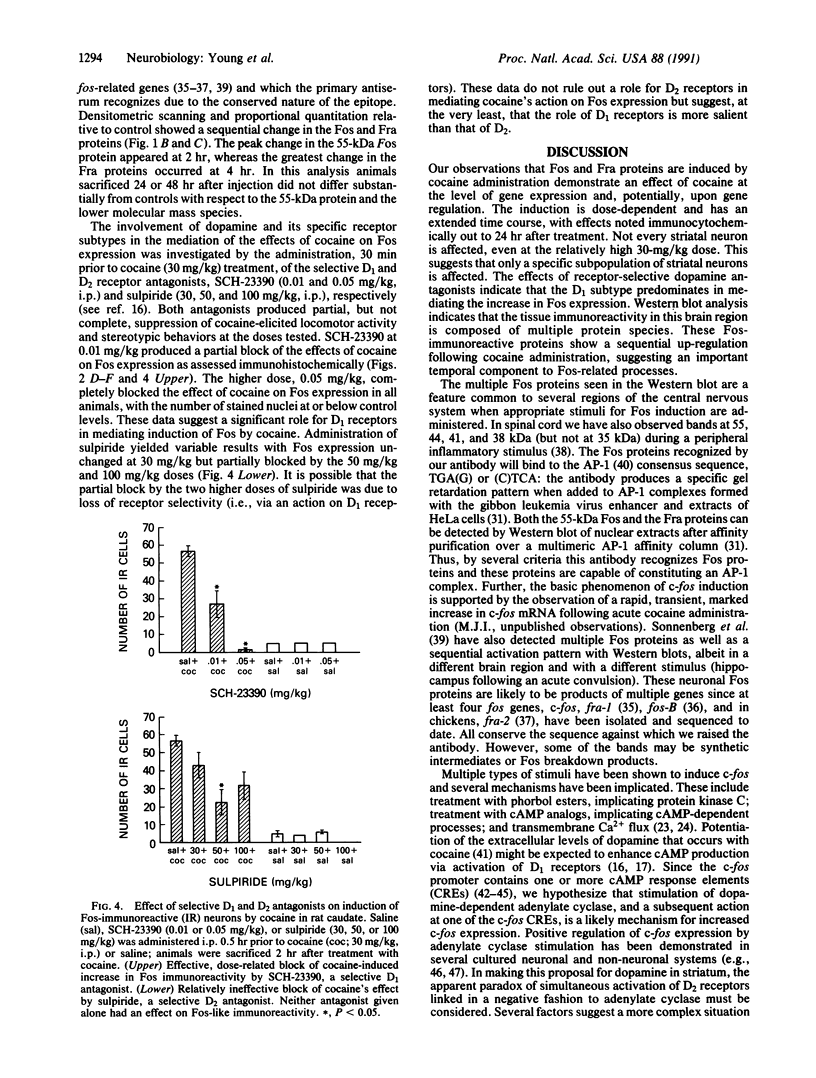
Abstract
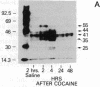
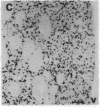
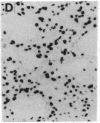
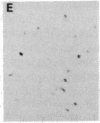
The protooncogene c-fos produces a phosphoprotein, Fos, which regulates gene transcription processes. In neuronal systems, Fos has been proposed to couple synaptic transmission to changes in gene expression by acting in the cell nucleus in concert with other proteins to form complexes in the promoter regions of target genes. We report here that the acute administration of a single dose of the indirect-acting dopaminergic agonist cocaine increases multiple Fos proteins in rat caudate nucleus. The increase is dose-dependent and is apparent immunocytochemically at 1 hr, maximal at 2 hr, and absent 48 hr after treatment. The increase seen immunocytochemically is composed of several molecular weight species as assessed by Western blotting of proteins from isolated striatal cell nuclei. Administration of the specific dopaminergic receptor antagonists sulpiride and SCH-23390 prior to cocaine support a significant role for D1 but not for D2 receptors in mediating this effect. These data indicate that D1 dopamine receptors are linked to a cellular immediate-early gene system(s) and suggest an action of cocaine at one or more levels of gene expression via modulation of transcriptional processes in activated cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz L. A., Riabowol K. T., Gilman M. Z. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989 Oct;9(10):4272–4281. doi: 10.1128/mcb.9.10.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Chio C. L., Hess G. F., Graham R. S., Huff R. M. A second molecular form of D2 dopamine receptor in rat and bovine caudate nucleus. Nature. 1990 Jan 18;343(6255):266–269. doi: 10.1038/343266a0. [DOI] [PubMed] [Google Scholar]
- Clark D., White F. J. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1(4):347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M., Bier-Laning C. M., Halford M. K., Ahlijanian M. K., Zahniser N. R. Dopamine, acting through D-2 receptors, inhibits rat striatal adenylate cyclase by a GTP-dependent process. Mol Pharmacol. 1986 Feb;29(2):113–119. [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- De Wit H., Wise R. A. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977 Dec;31(4):195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draisci G., Iadarola M. J. Temporal analysis of increases in c-fos, preprodynorphin and preproenkephalin mRNAs in rat spinal cord. Brain Res Mol Brain Res. 1989 Jul;6(1):31–37. doi: 10.1016/0169-328x(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978 Aug 1;180(3):545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Giardina S. L., Evans S. W., Gandino L., Robey F. A., Bonvini E., Longo D. L., Varesio L. Generation of a murine monoclonal antibody that detects the fos oncogene product. Anal Biochem. 1987 Feb 15;161(1):109–116. doi: 10.1016/0003-2697(87)90659-2. [DOI] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Goeders N. E., Smith J. E. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983 Aug 19;221(4612):773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- HERTING G., AXELROD J., WHITBY L. G. Effect of drugs on the uptake and metabolism of H3-norepinephrine. J Pharmacol Exp Ther. 1961 Nov;134:146–153. [PubMed] [Google Scholar]
- Hanson G. R., Merchant K. M., Letter A. A., Bush L., Gibb J. W. Methamphetamine-induced changes in the striatal-nigral dynorphin system: role of D-1 and D-2 receptors. Eur J Pharmacol. 1987 Dec 1;144(2):245–246. doi: 10.1016/0014-2999(87)90527-9. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Orlansky H., Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol. 1975 Apr 15;24(8):847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yoshikawa K., Kanamatsu T., Sabol S. L. Modulation of striatal enkephalinergic neurons by antipsychotic drugs. Fed Proc. 1985 Jun;44(9):2535–2539. [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Lin Y. S., Pearlberg J., Green M. R., Goodman H. M. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988 Oct;8(10):4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe B. K. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976 Dec;199(3):649–661. [PubMed] [Google Scholar]
- Kousvelari E., Louis J. M., Huang L. H., Curran T. Regulation of proto-oncogenes in rat parotid acinar cells in vitro after stimulation of beta-adrenergic receptors. Exp Cell Res. 1988 Nov;179(1):194–203. doi: 10.1016/0014-4827(88)90358-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li S. J., Sivam S. P., McGinty J. F., Jiang H. K., Douglass J., Calavetta L., Hong J. S. Regulation of the metabolism of striatal dynorphin by the dopaminergic system. J Pharmacol Exp Ther. 1988 Jul;246(1):403–408. [PubMed] [Google Scholar]
- Marriott S. J., Brady J. N. Enhancer function in viral and cellular gene regulation. Biochim Biophys Acta. 1989 Dec 17;989(2):97–110. doi: 10.1016/0304-419x(89)90037-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mocchetti I., De Bernardi M. A., Szekely A. M., Alho H., Brooker G., Costa E. Regulation of nerve growth factor biosynthesis by beta-adrenergic receptor activation in astrocytoma cells: a potential role of c-Fos protein. Proc Natl Acad Sci U S A. 1989 May;86(10):3891–3895. doi: 10.1073/pnas.86.10.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989 Nov;12(11):459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Nishina H., Sato H., Suzuki T., Sato M., Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci U S A. 1990 May;87(9):3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M., Brownstein M. J., Zamir N. On the origin of dynorphin A and alpha-neo-endorphin in the substantia nigra. Neuropeptides. 1984 May;4(3):193–199. doi: 10.1016/0143-4179(84)90100-8. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Takimoto M., Iadarola M., Holbrook N., Levens D. Distinct factors bind the AP-1 consensus sites in gibbon ape leukemia virus and simian virus 40 enhancers. J Virol. 1989 Apr;63(4):1737–1742. doi: 10.1128/jvi.63.4.1737-1742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner M. E., Jones B. E. Intravenous self-administration of cocaine and norcocaine by dogs. Psychopharmacology (Berl) 1980;71(1):83–89. doi: 10.1007/BF00433258. [DOI] [PubMed] [Google Scholar]
- Robertson H. A., Peterson M. R., Murphy K., Robertson G. S. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res. 1989 Dec 4;503(2):346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Ross S. B., Renyl A. L. Accumulation of tritiated 5-hydroxytryptamine in brain slices. Life Sci. 1967 Jul 1;6(13):1407–1415. doi: 10.1016/0024-3205(67)90188-9. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Visvader J., Ferland L., Mellon P. L., Verma I. M. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988 Dec;2(12A):1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sivam S. P. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989 Sep;250(3):818–824. [PubMed] [Google Scholar]
- Sonnenberg J. L., Macgregor-Leon P. F., Curran T., Morgan J. I. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989 Sep;3(3):359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981 Nov 26;294(5839):366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Tang F., Costa E., Schwartz J. P. Increase of proenkephalin mRNA and enkephalin content of rat striatum after daily injection of haloperidol for 2 to 3 weeks. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3841–3844. doi: 10.1073/pnas.80.12.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. Isolation of nuclei from liver and other tissues. Methods Enzymol. 1974;31:253–262. doi: 10.1016/0076-6879(74)31027-0. [DOI] [PubMed] [Google Scholar]
- Walters J. R., Bergstrom D. A., Carlson J. H., Chase T. N., Braun A. R. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987 May 8;236(4802):719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- White J. D., Gall C. M. Differential regulation of neuropeptide and proto-oncogene mRNA content in the hippocampus following recurrent seizures. Brain Res. 1987 Dec;427(1):21–29. doi: 10.1016/0169-328x(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]