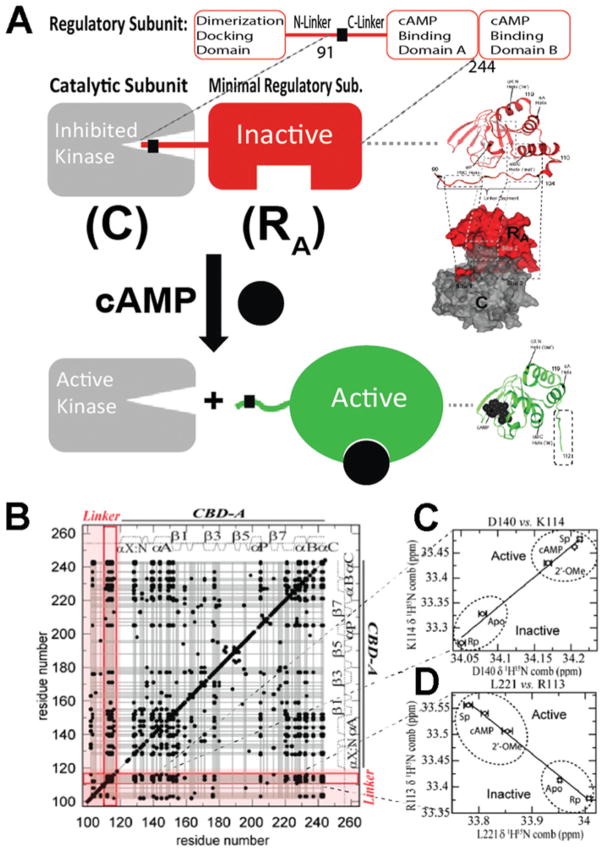
Figure 2. Role of the RA linker in PKA inhibition and allostery.
(A) cAMP-dependent activation of PKA. cAMP binding releases the inhibition of the catalytic subunit of PKA (C) by stabilizing a conformation of the regulatory subunit (R) that binds C only weakly. RA is defined as the minimal fragment of R that inhibits C in a cAMP-dependent manner. RA includes the linker and the adjacent CBD [i.e. RIα-(91–244)]. In the R–C complex, the inhibitory linker of R docks in the active site of C. Hence it is well-structured and clearly visible with conventional structure determination methods. In the R–cAMP complex, the linker of R is more solvent-exposed and flexible. Hence it is poorly resolved and not always visible through X-ray crystallography. However, the interactions between the flexible linker and the adjacent domain are effectively probed through NMR CHESCA, which establishes a residue–residue correlation matrix (B) based on pairwise inter-residue linear chemical shift relationships (C and D). The linker–domain CHESCA correlations are highlighted in red. Each black dot corresponds to a residue–residue combined chemical shift correlation with a Pearson’s correlation coefficient >0.98 in absolute value. (B–D) Adapted from [22]: Akimoto, M., Selvaratnam, R., McNicholl, E.T., Verma, G., Taylor, S.S. and Melacini, G. (2013) Signaling through dynamic linkers as revealed by PKA. Proc. Natl. Acad. Sci. U.S.A. 110, 14231–14236 with permission.