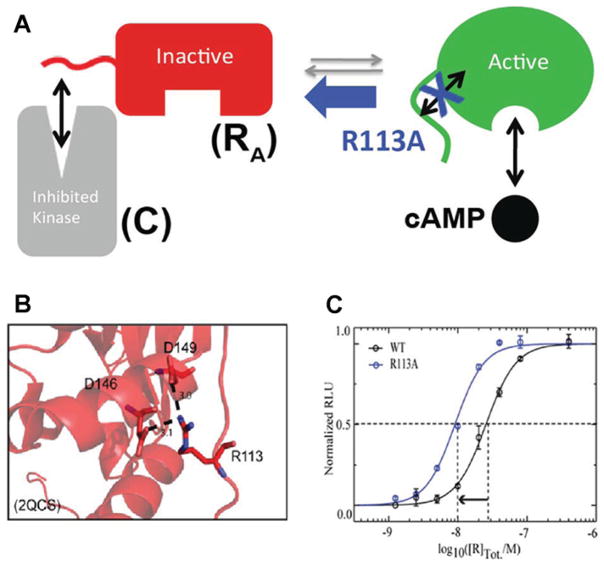
Figure 3. Role of the RA linker in conformational selection.
Apo RA samples both C-binding-competent conformations (‘inactive’) and cAMP-binding-competent conformations (‘active’). (A) Apo RA samples both C-binding competent conformations (‘inactive’) and cAMP-binding competent conformations (‘active’). The linker is one of the critical elements of RA that controls the position of this inactive versus active conformational equilibrium. When the interactions between the linker and the adjacent domain in the active conformation are weakened by a mutation (e.g. R113A) selected on the basis of CHESCA, the equilibrium shifts towards the C-binding-competent state, resulting in enhanced RA –C affinity (C), despite the fact that Arg113 is surprisingly forming salt bridges in the RA –C complex (B). (B and C) Adapted from [22]: Akimoto, M., Selvaratnam, R., McNicholl, E.T., Verma, G., Taylor, S.S. and Melacini, G. (2013) Signaling through dynamic linkers as revealed by PKA. Proc. Natl. Acad. Sci. U.S.A. 110, 14231–14236 with permission.