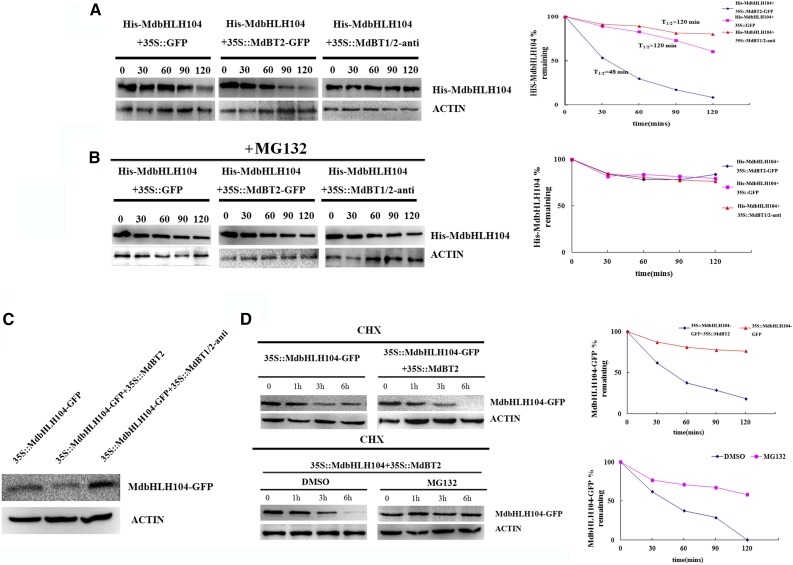
Figure 4.
The abundance and stability of the MdbHLH104 protein. A and B, Cell-free degradation assays of the purified recombinant His-MdbHLH104 protein in the protein extract of transgenic apple calli as labeled. Samples were incubated in the degradation buffer with or without proteasome inhibitor (50 μm MG132). His-MdbHLH104 levels were visualized by immunoblotting using the anti-His antibody. ACTIN was used as the loading control. Right, The half-life plot for the cell-free degradation assay of His-MdbHLH104 was calculated based on densitometric analyses of the immunoblots of His-MdbHLH104 and MdACTIN. C, The abundance of MdbHLH104 proteins in three transgenic apple calli as labeled. D, MdBT2-mediated destabilization of the MdbHLH104 protein is dependent on the ubiquitin/proteasome. 35S::MdbHLH104-GFP apple calli were transformed with or without the expression of MdBT2. At time 0, 250 μm CHX was added, and total proteins were extracted at the indicated times and then analyzed by immunoblotting using the anti-GFP antibody (top). 35S::MdbHLH104-GFP transgenic apple calli were transformed with or without 35S::MdBT2, and the resultant 35S::MdbHLH104-GFP+35S::MdBT2 transgenic calli were either treated with DMSO or MG132 for the indicated amounts of time. The total proteins were extracted at the indicated times and then analyzed by immunoblotting using the anti-GFP antibody (bottom). Quantification of the protein levels using Quantity One software is shown on the right.