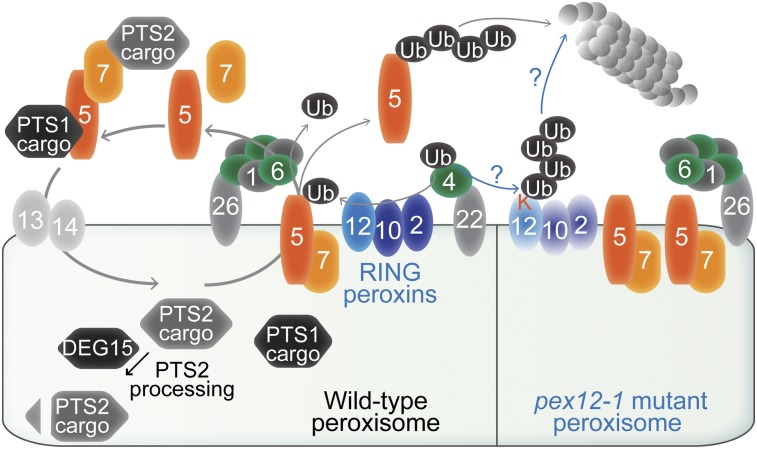
Figure 9.
A working model of peroxisome-associated ubiquitination machinery in wild type (left) and the pex12-1 mutant (right). Peroxins are numbered ovals. In wild type, the PEX5-PEX7-cargo complex docks at the peroxisome and delivers cargo into the matrix. The PEX4 ubiquitin-conjugating enzyme is suggested to work with the PEX2, PEX10, and PEX12 RING peroxins to ubiquitinate PEX5, which allows PEX5 (and PEX7) retrotranslocation by the PEX1-PEX6 ATPase complex for further rounds of cargo recruitment or proteasomal degradation. Although ubiquitination of PEX5 has not been directly shown in Arabidopsis, it is demonstrated in yeast (for review, see Platta et al., 2014) and is inferred from the low PEX5 levels in the pex6-1 mutant (Zolman and Bartel, 2004) that can be restored by treatment with a proteasome inhibitor (Kao and Bartel, 2015) and in pex6-1 pex4-1 (Ratzel et al., 2011) and pex6-1 pex2-1 (Burkhart et al., 2014) double mutants. The elevated PEX5 levels in pex2, pex10, and pex12 mutants (this work) also support the hypothesis that PEX5 is a substrate of these ubiquitin-protein ligases. Decreasing PEX4 function partially ameliorates pex12-1 defects, consistent with the possibility that the pex12-1 ectopic Lys substitution (K) is ubiquitinated, leading to pex12-1 degradation, RING peroxin complex destabilization, peroxisomal retention of receptors, and severe matrix protein import defects.