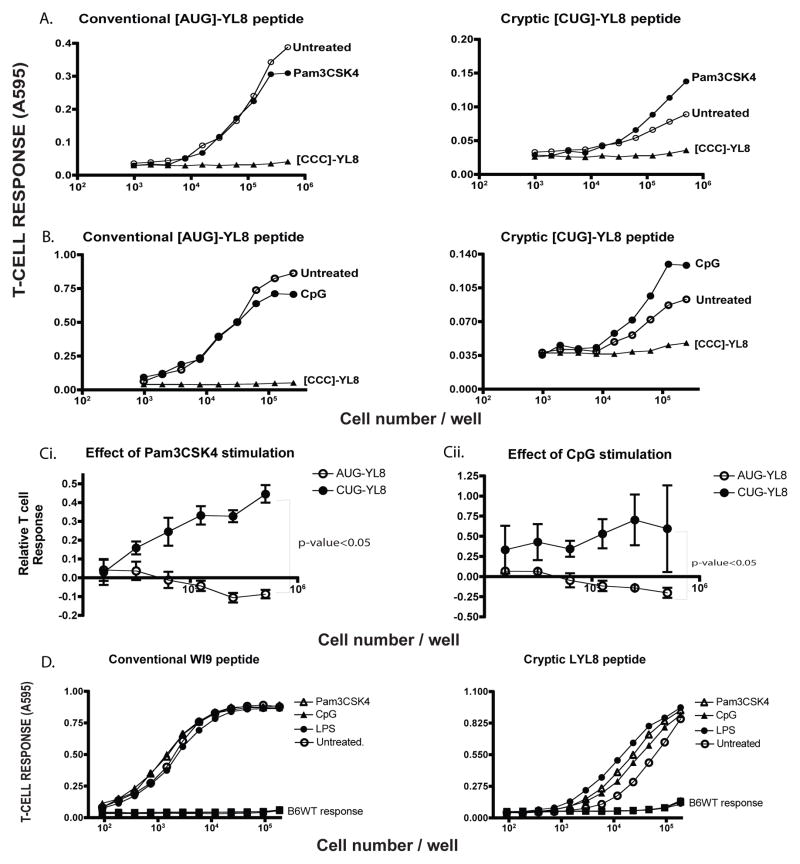
Figure 2. Toll-Like Receptor (TLR) agonists enhance cryptic peptide presentation.
A. The mRNA encoding MYL8 ([AUG]-YL8), LYL8 ([CUG]-YL8) peptides along with the negative control ([CCC]-YL8) were transfected into wild-type immortalized macrophages. After 3 hours, half of the transfected samples were treated with TLR2 agonist - Pam3CSK4 (1μg/mL) for 4 hours. The macrophages were then harvested and cultured with the lacZ inducible, BCZ103 T-cell hybridoma. The response of BCZ103 T-cells, shown on the Y-axis is a measure of the β-galactosidase activity determined by the conversion of the CPRG substrate to a colored product with an absorbance at 595 nm. The cell numbers in each well is indicated on the X-axis. Data is representative of 11 experiments. B. Identical experimental setup as in (A) except TLR9 agonist - CpG was used as the agonist. Y-axis indicates the BCZ103 T-cell response and X-axis indicates the cell number per well. Data is representative of 8 experiments. C. T-cell responses to the AUG-YL8 and CUG-YL8 peptides, upon Pam3CSK4 (i) stimulation or CpG stimulation (ii), were normalized to that of the untreated samples for 3 distinct experiments. An unpaired t-test (with Welch’s correction) was performed, comparing the 2 variables - p-value<0.05. D. Primary macrophages were isolated from the WI9.LYL8 transgenic mice and stimulated with indicated TLR agonists (1 μg/mL) for 6 hours and then cultured with either the T-cell hybridoma 11p9Z specific for the WI9 peptide or the LYL8 specific BCZ103 hybridoma. Macrophages from wild-type mice were used as a negative control. Y-axis indicates the T-cell response (11p9Z specific hybridoma response for conventional peptide and BCZ103 hybridoma response for the cryptic peptide) and the X-axis indicates the cell number per well.