Abstract
Studying the morphology of the arterial response to endovascular stent implantation, requires embedding the explanted stented artery in rigid materials such as poly (methyl methacrylate) to enable sectioning through both the in situ stent and the arterial wall, thus maintaining the proper anatomic relationships. This is a laborious, time consuming process. Moreover, the technical quality of stained plastic sections is typically suboptimal, and in some cases precludes immunohistochemical analysis. Here we describe a novel technique for dissolution of metallic and plastic stents that is compatible with subsequent embedding of “destented” arteries in paraffin, fine sectioning, major staining protocols and immunohistochemistry.
Keywords: stents, arterial tissue, histological processing, paraffin embedding, immunohistochemistry
1. Introduction
With more than 1 million devices deployed yearly worldwide [1], endovascular stents are by far the most widely used type of artificial implants in medical practice. Evolution of stent designs, especially pertaining to those which combine mechanical support with local drug elution from stent surface has led to a rapid expansion of indications for coronary and peripheral artery stenting and to ever decreasing rates of complications [2]. However, two clinical issues, in-stent restenosis (ISR) [3] and late stent thrombosis (LST) [4] present a formidable problem even with the state-of-art third generation drug-eluting stents (DES). While currently below 10% and 1%, respectively, ISR and LST carry significant medical and economic burden due to the voluminous use of stenting [5]. High-quality histological analysis allowing identification of specific cell types associated with stent struts, their physiological status and secretome is an essential tool for studying normal physiological healing response and the pathological reaction of the vessel wall to stent implantation. An inherent technical problem associated with histologic processing of stented arteries is the presence of metallic or polymeric struts encased in the neointima of long term implants. These elements are harder than surrounding vascular tissue, thus precluding successful sectioning of stented arteries embedded in paraffin. To enable sectioning, the stent-bearing arteries are embedded into a rigid media, such as poly (methyl methacrylate). Sectioning is carried out using either a precision saw with subsequent polishing [6, 7] or a heavy duty microtome equipped with a tungsten carbide knife [7]. This is a low-yield, laborious process requiring costly equipment and technical expertise. Moreover, the sections are often poor quality because the dragging momentum of the microtome results in strut displacement and tissue tears [7]. Additionally, immunohistochemical or immunofluorescence evaluation of sections is often challenging mostly due to excessive heat generated during embedding and sectioning [6]. Exposing arteries to harsh organic solvents to remove the polymeric imbedding media may also play a role in the impeded epitope binding by antibodies that readily work on paraffin embedded arterial tissue.
Recently, electrochemical dissolution of stents within the formalin-fixed arteries was reported by two groups [8, 9]. This method allows paraffin embedding of partially destented arteries thus facilitating subsequent sectioning and staining. Despite demonstrated proof of principal, it is difficult to envision routine use of electrochemical methods for stent dissolution since they are extremely laborious and destroy the proximal and distal ends of the stented segment. Moreover, since electric current stops when the circuit is broken, dissolution of stent struts is never complete, leaving islands of metallic wires that impede sectioning.
No prior study has investigated a direct chemical dissolution of metallic or polymeric stents. Metal alloys used for stent manufacture (316 L stainless steel, cobalt-chromium-tungsten-nickel alloy L-605, cobalt-platinum alloy, nitinol) are corrosion resistant. These materials withstand treatment with strong acids, due to a rapid formation of a surface layer consisting of metal oxides, which, being insoluble in acid, protects the underlying bulk of material from acidic dissolution. Interestingly, addition of relatively weak hydrofluoric acid (pKa=3.17) to nitric acid (pKa=−1.3) enables dissolution of stainless steel because of the high solubility of metal oxides in hydrofluoric acid [10]. Polyesters used for the fabrication of biodegradable stents and scaffolds, such as poly-L-lactide (PLLA) are readily soluble in non-polar organic solvents, dichloromethane and chloroform.
Here we report a novel method that provides complete stent dissolution in explanted, formalin-fixed stented-arteries with a fully formed neointima. We show that this “de-stenting” protocol enables paraffin embedding, preserves the morphology of the arterial tissue and, unlike conventional techniques using rigid imbedding media, is fully compatible with virtually all standard histological staining protocols and immunohistochemistry.
2. Materials and methods
2.1. Dissolution kinetics
Multilink 316L stainless steel stents (n=3; Laserage, Waukegan, IL), nitinol self-expanding stents removed from Smart Control Vascular Stent systems (n=3; Cordis, Hialeah, FL) and 1 mm × 0.1 mm × 8 mm coupons of L605 cobalt-chromium-tungsten-nickel alloy (n=3) cut from L605 foil (Goodfellow, Coraopolis, PA) were cleaned with isopropanol and chloroform and dried at 70°C. The weight of each sample was recorded. The samples were then individually exposed to 20 ml of a 1:1 (v/v) mixture of 3 N nitric acid and 12.8 N hydrofluoric acid at 28°C with mild shaking. After 5, 30, 90 and 360 min of incubation, the samples were removed from the acid solution, washed in water, wiped to dryness, weighed and placed back in the acid. The relative decrease of the samples weight was plotted over time to reflect dissolution kinetics. The dissolution of PLLA fibers (PL-32, 565 kDa, 65 μm diameter; Purac, Netherlands) in chloroform was similarly studied and presented.
2.2. Preparation of stents
Biodegradable stents were manufactured in-house (U.Texas, Dallas, TX) from PLLA resin, PL-32 (565kDa, PURAC Netherlands). PLLA was melt-extruded and drawn to a final diameter of 0.065 ± 0.01 mm [11]. The drawn fibers were then wound on a specially created jig to make the double opposed coiled stent. Axial reinforcing rods, made of the same drawn PLLA fiber, were inserted into the coil and fixed at the fiber overlaps. Stent crimping on the balloons of the PTA catheters (NuMed, Hopkinton, NY) was performed using a manual crimp tool (Model RJ25, Blockwise Engineering, Chicago, IL) to a final external diameter of 1.02 mm. The mounted stents were sterilized in 70% ethanol prior to use. Multilink stents (304 stainless steel grade; Laserage) were cleaned with isopropanol and chloroform and sterilized in 70% ethanol prior to mounting on PTA catheters (NuMed).
2.3. Animal experiments
Carotid stent angioplasty procedures were carried out in 400–450 g male Sprague-Dawley rats (n=10). After performing carotid balloon denudation with 3 passages of a 2F Fogarty catheter (Edwards LifeSciences, Irvine, CA) the stents (n=5 for each stent type) were deployed with PTA catheters (1.5 mm nominal diameter; NuMed) at 16 atm in the mid-segments of the left common carotid arteries as described in our previous publications [12–14]. The animals were euthanized 14 days after stent deployment. Bromodeoxyuridine (40 mg/kg) was injected intraperitoneally 6 hours before sacrifice. Harvested stented arterial sections were flushed with heparinized saline and fixed in 10% buffered formalin for a minimum of 48 hours. All animal studies were approved by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee and conducted in accordance with principles and procedures outlined in the NIH Guide for the Care and Use of Animals.
2.4. “De-stenting” procedures
2.4.1. Stainless steel stents
Formalin fixed arteries incorporating metallic stents were washed in running tap water for 2 hours, rinsed in distilled water and individually treated with 20 ml of a 1:1 (v/v) mixture of 3N nitric acid and 12.8 N hydrofluoric acid at 28°C with mild shaking for 5 hours. Completeness of stent dissolution was confirmed by microscopy using a dissecting microscope. The de-stented arteries were then washed in running tap water for 30 min, post-fixed in 10% neutral buffered formalin for additional 24 hours and routinely processed for paraffin embedding and sectioning.
We also tested feasibility of stent dissolution within archival plastic-embedded arteries to allow paraffin re-embedding. The uncut edge fragments of poly (methyl methacrylate)-embedded rabbit iliac arteries bearing stainless steel stents were deplastified with 2-methoxyethyl acetate (Aldrich) and hydrated through graded ethanol sequence. Strut dissolution was carried out in HNO3/HF formulation as described above. De-stented segments were then re-fixed for 24 hours in formalin and routinely embedded in paraffin.
2.4.2. PLLA stents
The formalin-fixed arteries incorporating PLLA stents were washed in running tap water for 2 hours, and dehydrated in graded ethanol and xylene. After the second xylene change, PLLA stent-deployed arteries were exposed to chloroform at 28°C with mild shaking for 20 min. Completeness of stent dissolution was confirmed microscopically as above. The de-stented arteries were then transferred to 100% xylene and routinely processed for paraffin embedding and sectioning.
2.5. Immunohistochemistry and special stains
The slides were deparaffinized and boiled in a pressure cooker with antigen retrieval buffer (H-3300; Vector Labs, Burlingame, CA) for 3–5 min. The sections were then blocked with 10% horse serum for 20 min, followed by application of either a primary antibody or non-immune IgG at room temperature for 1 hour. After PBS washing, the sections were consecutively treated with the secondary biotinylated anti-mouse (BA-2001, Vector Labs) or anti-rabbit (BA-1100, Vector Labs) antibodies (1:100–1:200 dilution; 45 min), Vectastain Elite ABC reagent (PK-6100, Vector Labs) and peroxidase substrate (ImmPact DAB; SK-4105, Vector Labs). Finally, the sections were counterstained with Gill 3 hematoxylin (5 and 40 seconds for the PLLA de-stented and stainless steel de-stented samples, respectively) and mounted. Non-immune special stains, Verhoeff-van Gieson elastic stain and Masson’s trichrome staining were carried out according to the instructions provided by the manufacturers of the respective kits (HT25A; Sigma-Aldrich, St. Louis, MO and 25088; Polysciences, Warrington, PA). Digital images were captured under 40–200× magnification with a Nikon Eclipse 80i microscope.
3. Results
3.1. Dissolution kinetics of non-implanted stents
Optimization experiments (not reported here) have established the optimal molar ratio of 3–3.4 between hydrofluoric and nitric acid for dissolution of 316L grade stainless steel and investigated the stainless steel dissolution rate as a function of nitric acid concentration. The lowest nitric acid concentration capable of stainless steel dissolution was chosen to determine dissolution kinetics of actual stent samples manufactured from 316L stainless steel and nitinol. Since no stents manufactured from cobalt-chromium alloy, L605, were made available for us, we chose to use strips of L605 alloy foil cut to approximate the dimensions of stent struts. While nitinol and stainless steel stent were completely dissolved in the acid mixture in 30 and 360 min, respectively, the weight of L605 strips was only marginally affected (Table 1). PLLA fibers were completely dissolved in chloroform in 30 min (Table 1).
Table 1.
Dissolution kinetics of selected material used for stent manufacture.
A relative decrease of the sample mass (100%) upon exposure to the nitric/hydrofluoric acid mixture (316 grade stainless steel, nitinol and Co-Cr alloy L-605) or to chloroform (PLLA) as a function of solvent immersion time.
| Material | t=0 min | t=5 min | t=30 min | t=90 min | t=180 min | t=360 min |
|---|---|---|---|---|---|---|
| Co-Cr alloy (L605) | 100 | 99.8±0.3 | 99.6±0.3 | 99.6±0.3 | 99.5±0.4 | 99.3±0.2 |
| Nitinol | 100 | 38.2±3.6 | dissolved | dissolved | dissolved | dissolved |
| Stainless steel (316L) | 100 | 98±0.6 | 78.8±0.9 | 57.9±0.7 | 38.8±1 | dissolved |
| PLLA | 100 | 12±2.3 | dissolved | dissolved | dissolved | dissolved |
3.2. Stent dissolution of arterial samples explanted from the experimental animals
Only stainless steel and PLLA stents sized to fit rat carotid arteries were available to us and were thus used in in vivo experiments. All animals survived the stent deployment procedure and were euthanized 14 days after stent implantation. The de-stenting protocol for both metal and PLLA stent-implanted arteries was monitored using a dissecting microscope (Fig. 1). The maximum time required to completely dissolve stent elements embedded into the vessel wall was 15 and 240 min for the PLLA and stainless steel stents, respectively. No polymer or metal inclusions were identified in the processed PLLA and stainless steel stent-treated arteries upon careful visual examination using a dissecting microscope or during subsequent sectioning. No apparent changes in arterial tissue integrity and structure occurred upon the acid mixture treatment; however, a yellowish discoloration of tissue was noticed (Fig. 1A and C). Overall appearance of the arteries did not change upon chloroform exposure (Fig, 1B and D). The acid-treated arterial samples required additional formalin fixation after stent dissolution to ensure adequate cross-linking, since formalin-introduced cross-links do not withstand strong acidic environment [15].
Fig. 1.
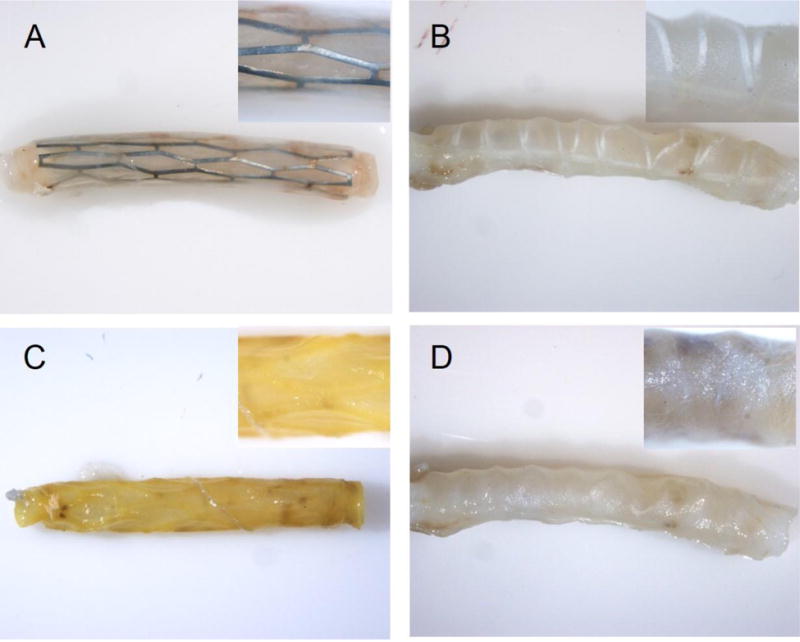
Chemical dissolution of stents in situ.
Explanted rat carotid arteries implanted with stainless steel (A, C) or PLLA (B, D) stents are shown before (A, B) and after (C, D) stent dissolution. Original magnification is 8× (insets are 40×).
3.3. H&E and special staining techniques of “de-stented” paraffin-embedded arterial sections
To ascertain morphological preservation of arterial tissue under the chemical conditions expected to attain complete elimination of stent struts, non-stented balloon-injured segments of rat carotid arteries were either 1) paraffin-embedded as routine; 2) exposed to a 1:1 mixture of 3N nitric and 12.8N hydrofluoric acids at 28°C with mild shaking for 5 hours, or 3) processed through the dehydration sequence up to the first xylene change, and then exposed to chloroform at 28°C with mild shaking for 30 min, washed with xylene (5 min) followed by routine paraffin embedding. Verhoeff-van Gieson stained arterial sections originated from the vascular tissue differently treated in respect to the acid/chloroform exposure do not demonstrate significant morphological dissimilarities (Supplementary Data, Fig. 1).
We verified compatibility of the de-stenting protocols applied to stent-bearing arteries with subsequent use of 3 common staining techniques widely used for histological assessment of vascular tissue. (Fig. 2A–G and 3A–G). Verhoeff-van Gieson (VVG) elastic stain by dyeing medial elastin fibers black is essential for clear delineation of arterial compartments and thus is a preferred technique for morphometric endpoints. Low magnification (40×) images of VVG-stained arterial sections from the animals treated with stainless steel (Fig. 2A) and with PLLA (Fig. 3A) stents demonstrate intact structure of the stented arteries and adjacent muscle, nerve and connective tissue elements. Massive neointimal thickening is readily identifiable as the tissue located inside of the innermost medial elastin layer (internal elastic lamia). The empty spaces left by the dissolved stent struts are typically encased in neointima and do not communicate with the lumen. Higher magnification (100× and 200×) images (Fig. 2B–C; Fig. 3B–C) allow for more detailed morphological characterization showing, for example better apposition of metallic compared to polymeric stents as evidenced by the formation of neointima between the PLLA strut and the medial layer (Fig. 3C). Masson’s trichrome stain, in addition to substantiating morphological integrity of stented arteries provides important information on the extent and condition of connective tissue, primarily collagen (stained blue). As can be appreciated from comparison of Fig. 2D–F and 3D–F, the amount of Masson’s-stained collagen is significantly higher in the arteries implanted with the PLLA stents. Extracellular matrix comprises up to 90% of the neointimal volume and thus even a small difference in the collagen production/breakdown ratio may significantly impact in-stent restenosis. Our future studies will investigate whether dissimilar Masson’s staining of metal and polymeric stent-treated arterial tissue reflects a true difference in collagen abundance or is an artifact related to partial collagen degradation during acidic stent dissolution. Hematoxylin and eosin staining of both metal (Fig. 2G) and polymeric stent (Fig. 3G)-deployed rat carotid arteries shows the presence of marked neointimal thickening and preserved structural integrity of all arterial layers. Some red blood cells are present in the arterial lumen and in the spaces left by the dissolved struts in the neointima (Fig. 2G). As with other stains, the intensity of hematoxylin component is reduced in the acid treated arterial samples, most probably due to hydrolysis of nucleic acids.
Fig. 2.
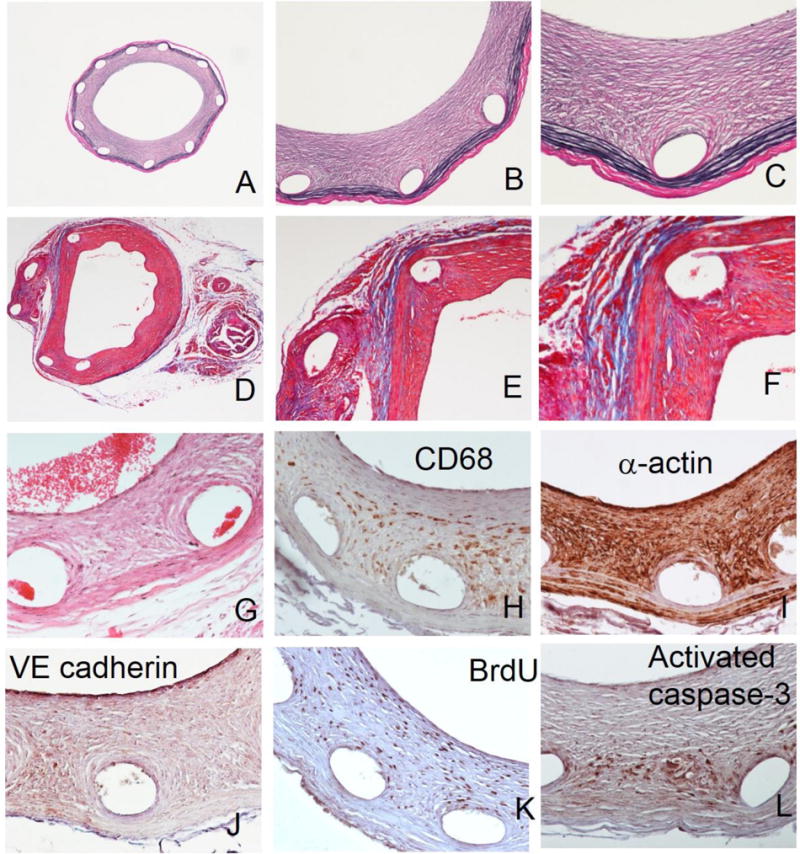
Special staining and immunohistochemistry of paraffin sections prepared following metallic stent dissolution.
Representative arterial sections stained according to Verhoeff-van Gieson (A–C), Masson’s trichrome (D–F) and H&E (G) protocols. Typical results of immunohistochemical staining with primary antibodies chosen to detect CD68-positive macrophages (H), α-actin-positive SMC (I), VE cadherin-positive endothelial cells (J), proliferating BrdU-positive cells (K) and activated caspase 3-positive cells undergoing apoptosis (L). Original magnifications: 2A, 2D – 40x; 2B, 2E – 100x; 2C, 2F–2L – 200x.
Fig. 3.
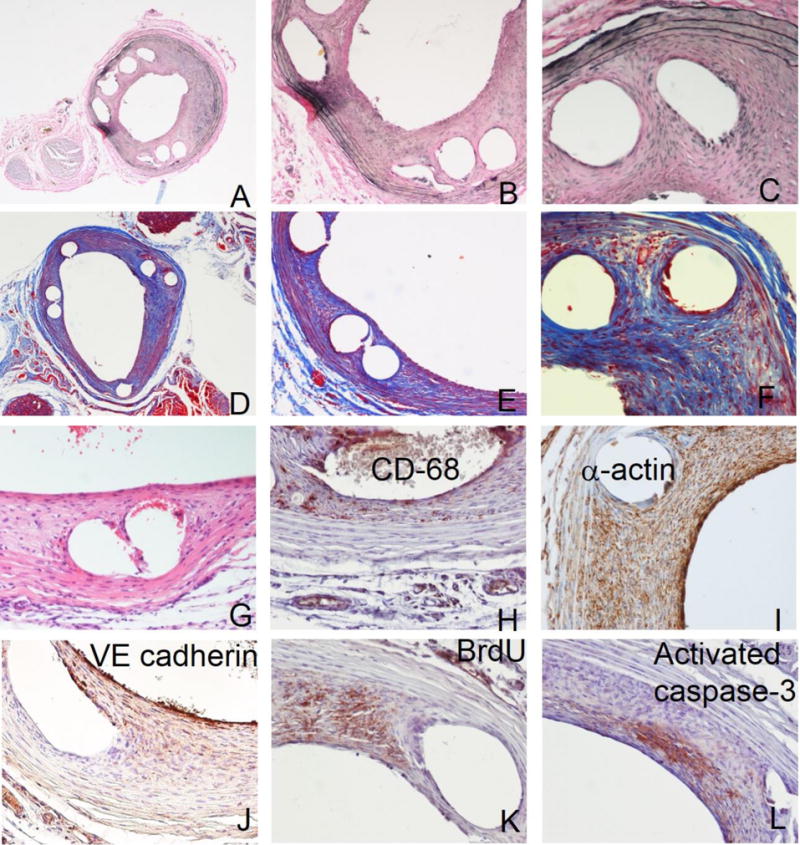
Special staining and immunohistochemistry of paraffin sections prepared following PLLA stent dissolution.
Representative arterial sections stained according to Verhoeff-van Gieson (A-C), Masson’s trichrome (D–F) and H&E (G) protocols. Typical results of immunohistochemical staining with primary antibodies chosen to detect CD68-positive macrophages (H), α-actin-positive SMC (I), VE cadherin-positive endothelial cells (J), proliferating BrdU-positive cells (K) and activated caspase 3-positive cells undergoing apoptosis (L). Original magnifications: 3A, 3D – 40x; 3B, 3E – 100x; 3C, 3F–3L – 200x.
Verhoeff-van Gieson staining was also performed on uncut edges of archival (6 year shelf storage) plastic embedded rabbit iliac samples that were re-embedded in paraffin after strut removal. The staining results demonstrate better preserved morphology in the paraffin re-embedded samples than in the original plastic sections derived from the same arteries (Supplementary Fig. 2).
3.4. Immunohistochemistry of “de-stented” paraffin-embedded arterial sections
Immunohistochemistry is instrumental in elucidating the intricate relationship between the cell types implicated in vascular response to stent-induced trauma [16]. Additionally, it provides unique information about crucial physiological processes, such as proliferation [16, 17] and apoptosis [16, 18] driving the expansion of neointima. We validated feasibility and quality of immunostaining on sections prepared from stented arteries exposed to strong acids or to chloroform as necessitated by the stent dissolution requirements, using primary antibodies to the following targets: CD68-positive macrophages (Fig. 2H and 3H), α-actin positive SMC (Fig. 2I and 3I), VE-cadherin positive endothelial cells (Fig. 2J and 3J), BrdU (proliferation marker; Fig. 2K and Fig. 3K), and activated caspase-3 (apoptosis; Fig. 2L and 3L). The expected staining patterns were detected. Specifically, CD68-positive cells were primarily found in neointima around the struts and in adventitia (Fig. 2H, 3H). SMC were detected in neointima and media with relatively low adventitial staining (Fig. 2I, 3I). A discontinuous layer of newly formed endothelium was demonstrated at the luminal surface of neointima and in some cases on the interface of a dissolved stent strut and overgrowing neointima (Fig. 2J, 3J). Proliferating cells positively stained for BrdU were mostly detected around struts and in the innermost part of neointima (Fig. 2K, 3K). There were relatively low numbers of cells undergoing apoptosis as reflected by the activation of the caspase pathway. These cells were primarily located in the vicinity of struts (Fig. 2L, 3L). The reliability of the immunostaining results was confirmed on all occasions by the lack of staining when a primary antibody was substituted for non-immune IgG, and by the identical staining pattern obtained on the adjacent sections (Supplementary Data, Fig. 2).
4. Discussion
Plastic embedding currently used for the histological processing of stented arteries is a laborious, low throughput methodology, which often yields poor quality material that precludes advanced microscopic analysis. The presented studies describe a novel tissue processing method that is based on in situ dissolution of stent structural elements in the formalin fixed stented arteries with subsequent routine paraffin embedding and sectioning. We found that formalin fixed tissue withstands treatment with concentrated acids and non-polar solvents without compromising tissue integrity and antigenicity.
The dissolution profile of pure metals and their alloys in acidic solutions was previously investigated from the metallurgical standpoint [10] providing us with the starting information regarding the composition and the concentration range of acidic mixtures capable of stainless steel stent dissolution. We furthered this prior knowledge with experiments designed to establish the specific composition of a “cocktail” which is effective in stent dissolution while not causing substantial damage to the biological sample. Acceptable general morphology and preserved antigenicity in formalin-fixed samples upon exposure to mineral and organic acids was previously validated in bone tissue in the course of development of decalcification protocols [19, 20]. Our results expand these data by showing that neither a HNO3/HF mixture by itself, nor the high concentrations of locally released metal ions precludes downstream morphological and immunohistochemical analyses of the stented arterial samples.
Acid exposure is well known to cause DNA degradation via separation of DNA strands, loss of bases through the breakage of glycosidic bonds and depolymerization through the hydrolysis of phosphodiester bonds. Most nuclear stains and counterstains, such as hematoxylin, DAPI and Hoechst 33258 bind to intact DNA. Therefore, nuclear stains produce much less contrast in the sections generated from the acid-treated tissue. To some extent, this nuclear staining defect may be compensated by prolonged staining times. Nevertheless, acidic stent dissolution is a poor choice for the staining experiments requiring precise delineation of nuclei. Partial degradation of collagen is another possible artefact introduced by the acidic exposure. Partial nucleic acid and protein degradation affects molecular and cell biology endpoint analysis techniques, such as PCR, immunoblotting, and in-situ hybridization, which are commonly used in restenosis studies. Undoubtedly, additional experiments will be necessary to determine the feasibility of these techniques in chemically “destented” arteries. The most important limitation identified by us is that the studied acid formulation was unable to dissolve cobalt-chromium (L605 foil strips). Currently, a sizable portion of the stent market is devices utilizing this alloy. However, it is quite possible that a systematic approach for the optimization of acidic mixture composition and reaction conditions will yield a formulation capable of dissolving L605 stents.
5. Conclusions
By demonstrating technical feasibility of complete dissolution of metallic and polymeric stents encased in vascular tissue, the present study introduces a novel approach for the paraffin processing of stented arteries for morphologic analyses. This straightforward technique produces better quality sections than most currently used methods and is compatible with the staining and immunohistochemistry protocols used to study pathophysiology of in-stent restenosis.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Frederick Schoen, Department of Pathology, Brigham and Women’s Hospital, for his review and comments. This work was supported by a grant from the W.W. Smith Charitable Trust to IF (#H-1402), the Children’s Hospital of Philadelphia Cardiac Center Research grant (IF) and by the NIH NHLBI grant R01-HL111118 (MC). Additional research support was provided by both the William J. Rashkind Endowment and Erin’s Fund of the Children’s Hospital of Philadelphia (RJL). PTA catheters were kindly provided by NuMed (Hopkinton, NY). Authors wish to acknowledge secretarial assistance of Ms. Susan Kerns.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Smits PC, Vlachojannis GJ, McFadden EP, Royaards KJ, Wassing J, Joesoef KS, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice: The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularization in Daily Practice) JACC Cardiovasc Interv. 2015;8:1157–65. doi: 10.1016/j.jcin.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Minha S, Pichard AD, Waksman R. In-stent restenosis of drug-eluting stents. Future Cardiol. 2013;9:721–31. doi: 10.2217/fca.13.45. [DOI] [PubMed] [Google Scholar]
- 4.Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–21. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 5.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J. 2015;36:3320–31. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik N, Gunn J, Holt CM, Shepherd L, Francis SE, Newman CM, et al. Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry, and transmission electron microscopy. Heart. 1998;80:509–16. doi: 10.1136/hrt.80.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rippstein P, Black MK, Boivin M, Veinot JP, Ma X, Chen Y-X, et al. Comparison of processing and sectioning methodologies for arteries containing metallic stents. J Histochem Cytochem. 2006;54:673–81. doi: 10.1369/jhc.5A6824.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw SH, Kennedy L, Dexter DF, Veinot JP. A practical method to rapidly dissolve metallic stents. Cardiovasc Pathol. 2009;18:127–33. doi: 10.1016/j.carpath.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Samra A, Brown K, Leung C, Meredith A, Allard M. Method evaluation of in situ dissolution of metallic coronary artery stent. J Histotechnol. 2010;33:182–6. [Google Scholar]
- 10.Covino BSJ, Scalera JV, Driscoll TJ, Carter JP. Dissolution behavior of 304 stainless steel in HNO3/HF mixtures. Metallurg Transact A. 1986;17A:137–49. [Google Scholar]
- 11.Welch TR, Eberhart RC, Chuong CJ. The influence of thermal treatment on the mechanical characteristics of a PLLA coiled stent. J Biomed Mater Res Part B, Appl Biomater. 2009;90:302–11. doi: 10.1002/jbm.b.31286. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein I, Alferiev I, Bakay M, Stachelek SJ, Sobolewski P, Lai M, et al. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation. 2008;117:2096–103. doi: 10.1161/CIRCULATIONAHA.107.746412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishbein I, Forbes SP, Adamo RF, Chorny M, Levy RJ, Alferiev IS. Vascular gene transfer from metallic stent surfaces using adenoviral vectors tethered through hydrolysable cross-linkers. J Vis Exp. 2014:e51653. doi: 10.3791/51653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slee JB, Alferiev IS, Nagaswami C, Weisel JW, Levy RJ, Fishbein I, et al. Enhanced biocompatibility of CD47-functionalized vascular stents. Biomaterials. 2016;87:82–92. doi: 10.1016/j.biomaterials.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sompuram SR, Vani K, Messana E, Bogen SA. A molecular mechanism of formalin fixation and antigen retrieval. Am J Clin Pathol. 2004;121:190–9. doi: 10.1309/BRN7-CTX1-E84N-WWPL. [DOI] [PubMed] [Google Scholar]
- 16.Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–63. doi: 10.1093/cvr/cvt115. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615–21. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- 18.Erl W. Statin-induced vascular smooth muscle cell apoptosis: a possible role in the prevention of restenosis? Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:135–44. doi: 10.2174/1568006043586134. [DOI] [PubMed] [Google Scholar]
- 19.Athanasou NA, Quinn J, Heryet A, Woods CG, McGee JO. Effect of decalcification agents on immunoreactivity of cellular antigens. J Clin Pathol. 1987;40:874–8. doi: 10.1136/jcp.40.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Chavez SA, Pacheco-Tena C, Macias-Vazquez CE, Luevano-Flores E. Assessment of different decalcifying protocols on Osteopontin and Osteocalcin immunostaining in whole bone specimens of arthritis rat model by confocal immunofluorescence. Int J Clin Exp Pathol. 2013;6:1972–83. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


