Abstract
There is a higher incidence of allergic conditions among children living in industrialized countries than those in developing regions. One explanation for this is reduced neonatal exposure to microbes and the consequent lack of immune stimulation. Sensitivity to cockroach allergen is highly correlated with the development of severe asthma. In this study, we determined that an antibody to microbial α-1,3-glucan binds an Enterobacter species and cockroach allergen. Neonatal, but not adult, mice immunized with this α-1,3-glucan-bearing Enterobacter (MK7) are protected against cockroach allergy. Following exposure to cockroach allergen, α-1,3-glucan-specific IgA-secreting cells are present in the lungs of mice immunized with MK7 as neonates, but not in the lungs of those immunized as adults. Mice that are unable to generate anti-α-1,3-glucan IgA antibodies were immunized with MK7 as neonates and were no longer protected against cockroach allergy. Thus, neonatal, but not adult, exposure to α-1,3-glucan results in suppressed development of cockroach allergy via pulmonary α-1,3-glucan-specific IgA-secreting cells.
Introduction
The childhood risk of developing an autoimmune or allergic condition is increasing more rapidly in industrialized, compared to developing, regions of the world (1-3). A theory termed the “Hygiene Hypothesis” suggests that this increase is the result of decreased exposure to microbes among children living in these developed environments (4-6). A favored paradigm associated with the hygiene hypothesis is that type 1 helper (TH1) T cells stimulated by early microbial exposure suppress the development of type 2 helper (TH2)-associated allergic disease (7, 8). However, little empirical evidence supports the long-term maintenance of TH1 cells or the ability of this T cell subset to suppress allergic disease development throughout the lifetime of the individual. In fact, early microbial exposure may have a multitude of effects on the developing immune system. We and others have described examples of significant alterations to the developing B cell repertoire following neonatal antigen exposure in mice (9).
During neonatal B cell lymphopoiesis we observe intraclonal competition between distinct emerging B cell clones that express different B cell receptors (BCRs) but have similar antigen specificity. Depending on the time after birth, exposure to particular bacterial antigens causes these emerging B cell clones to wax and wane according to their B cell receptor specificity, such that some will remain dominant in the adult repertoire, where others will become minor players (9-11). In addition, neonatal antigen exposure may be able to stimulate innate-like B1 cells that are enriched within the neonatal spleen (12) and have unique functions such as the ability to reside within the pleural and peritoneal cavity, contribute to serum IgM production, self renew (13), and readily switch to IgA (14). Exposure to particular bacterial antigens during early life can alter the numbers of these developing clones and permanently select them in to the adult repertoire (9). We have previously demonstrated that altered antigen-specific B cell responses, following neonatal immunization with bacterial vaccines containing polysaccharide or phospholipid moieties shared by Aspergillus fumigatus fungi or house dust mite (HDM), suppress allergic disease development in adult animals (15, 16).
Cockroaches are a prominent source of allergens, and cockroach sensitivity affects ∼26% of the US population (17). About 40%-60% of patients with asthma are sensitized to the German cockroach (Blattella germanica) (18, 19). The major allergenic proteins from the German cockroach, Bla g 1 and Bla g 2, are found in the insect feces, saliva, and exoskeletons (20, 21). Cockroach sensitization among children is often associated with urban environments (18, 22), low socioeconomic status (17, 23), and is more frequent within the African American community (23). Children that are skin-prick test positive for cockroach allergens are more likely to have difficult-to-control asthma (18, 19) and are three- to four-times more likely to visit healthcare providers or the emergency room due to an asthmatic event (24) than those suffering from other allergies. The allergenic potential of cockroach-associated proteins, such as Bla g 1 and Bla g 2, is well described (21, 25) and these proteins are used in immunotherapy treatments (26, 27). However, the association of conserved carbohydrates (28) and lipids (29) on allergen-bearing particles is often overlooked and the involvement of these epitopes in modulating the allergic immune response is not well appreciated.
Here, we show that that cockroach particles contain anti-α-1,3-glucan-reactive epitopes. α-1,3-glucans, also referred to as α-1,3-dextran (DEX) (30), are also expressed by fungi (31, 32) and some types of bacteria (33-35). The largely T-independent mouse antibody response to α-1,3-glucan is well-characterized and mostly consists of IgM, IgG3, and IgA isotypes (10, 30, 36-38). Additionally, B cells with specificity for α-1,3-glucan are enriched within the marginal zone and B1b cell populations (37). In this study, we examined the effects of neonatal exposure to α-1,3-glucan on the subsequent development of an allergic response to cockroach allergen. Immunization of neonates with α-1,3-glucan, either as a bacteria-derived vaccine or a purified polysaccharide, results in the localization of α-1,3-glucan-specific IgA-secreting cells to the lung and a dramatic reduction of allergic responses to cockroach allergen in mice challenged with cockroach allergen as adults.
Materials and Methods
Animals
C57BL/6 mice and triple congenic mice (Gpi1a Thy1a Igha) were originally obtained from Jackson Laboratory. Such congenic mice will be referred to in this study as B6.Igh-Ca. IgA-deficient mice (39) that expressed the Igh-Ca haplotype on a C57BL/6 background were obtained from Charles Elson III at the University of Alabama at Birmingham. C57BL/6 mice (Igh-Cb Igh-2+/+) were crossed to IgA-deficient mice (Igh-Ca Igh-2-/-) to generated F1 mice that do not make anti-α-1,3-glucan IgA (Igh-2a-/-). All mice were maintained under specific pathogen-free conditions using approved animal protocols from the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Cockroach allergen particulate preparation
Powdered, dried, acetone-defatted German cockroach (RMB46P, Greer Laboratories) was sonicated at 12 watts at 4°C in 30-minute increments for 15 hours before passage through a 10-micron filter. Particulate size was determined to be smaller than 0.5μm by flow cytometric comparison with 0.1 μm to 1 μm latex beads as reference particles (Sigma LB1-LB30). Cockroach particulates were pelleted at 10,000 rpm for 30 minutes prior to lyophilization. The lyophilized particulates were resuspended in sterile PBS at 10 mg/mL and stored at -80°C or diluted to 100 μg/mL then stored at 4°C until administration to mice.
Blattella germanica husbandry and dissection
Male and female Blattella germanica (German cockroach) were purchased from Carolina Biologics and reared at the University of Alabama at Birmingham. Roaches are maintained at 70°C and 80% humidity and are supplied with water, commercial dog food, and potato slices ad libitum. For antibody-binding studies, female roaches carrying an egg case (ootheca) were dissected into head, thorax, wings, abdomen, legs, ootheca, nymphs, and alimentary canal (A.C.) and these parts were homogenized separately. Fecal pellets were also collected from the colony and bacteria from the alimentary canal (A.C) were enriched in LB and THY broths. Cockroach components or bacteria were stained with a monoclonal antibody against α-1,3-glucan (A16) as described in Antibody Reagents and Flow Cytometry.
Enterobacter vaccines and immunizations
Enterobacter cloacae strains cBAN (α-1,3-glucan-deficient) (40) or MK7 (α-1,3-glucan-bearing) (33) were grown to mid-log phase in Lysogeny broth (LB) at 37°C in 5% CO2. Bacteria were washed, fixed with 1% paraformaldehyde (PFA) for 12 hours, then resuspended in sterile PBS and stored at -80°C until use. Neonatal (7- to 8-day old pups) or adult (8- to 10-week old) mice from the same litter (littermate) were immunized intraperitoneally (i.p) with 5×107 of PFA-fixed cBAN or MK7 Enterobacter strains or treated with PBS. Alternatively, neonatal (7- to 8-day-old) littermate pups were immunized i.p with 25μg β-1,3-glucan (Laminarin, Sigma) or α-1,3-glucan (gift from A. Jeanes, USDA) or treated with PBS.
In vivo intratracheal challenge and the cockroach allergy model
For intratracheal challenge, 8 to 10 week old (adult) mice were anesthetized with 3-5% isoflurane and then immobilized on a vertical board with suture string looped around the upper incisors. The tongue was extended using blunt-end forceps, liquid was pipetted into the oral cavity, and the nares were manually plugged to ease inhalation of the liquid suspension. Adult mice were challenged intratracheally (i.t.) with 5μg processed cockroach particulate allergen resuspended in 50μL PBS. Mice were rested for 7 days before being challenged i.t. daily for 5 consecutive days with 5μg cockroach in 50μL PBS. Following the last challenge with cockroach allergen, mice were rested for 2 days before euthanization.
Tissue and leukocyte collection and preparation
Following euthanization, tracheae were cannulated to extract cellular infiltrates from the bronchoalveolar space via a 5-milliliter lavage with PBS, from which the first milliliter was reserved for measuring antibodies. Mice were perfused by cardiac puncture with 1% heparin in PBS prior to lung or spleen removal. Lungs were minced and treated with 1 mg/mL collagenase (Sigma) + 50U DNAse (Sigma) in 5mLs HBSS for 40 minutes at 37°C followed by lymphocyte separation (Cellgro). MedLN and spleen cells were collected by mechanical isolation. Bone marrow was flushed from two femurs and two tibia per mouse using a 25-gauge needle. Spleen and bone marrow cell suspensions were subjected to ammonium-chloride-potassium red blood cell lysis (Gibco). All recovered cell populations were manually enumerated using a hemocytometer.
Enzyme-Linked Immunospot Assay (ELISPOT)
High-binding flat-bottomed EIA/RIA plates (Costar) were coated with 2 μg/ml of purified α-1,3-glucan to quantify α-1,3-glucan-specific IgM-, IgA-, and IgG3-secreting cells, or with 1 μg/ml anti-mouse IgM (Southern Biotechnology) or anti-mouse IgA (Southern Biotechnology) to identify total IgM- or IgA-secreting cells, respectively. Plates were coated overnight at 4°C, and then blocked for 6 hours at 37°C with a 1% gelatin in PBS solution. Single-cell suspensions were prepared in RPMI-1640 (Gibco) supplemented with 2% Fetal Bovine Serum (FBS) (Life technologies), and 5×105 bone marrow cells or 2×106 cells from the lung or spleen were added to one well of the plate and serially diluted with the same media. Plates were then incubated for 18 hours at 37°C. Cells were then lysed with 0.05% Tween-20 in water and plates were washed three times with 0.05% Tween-20 in PBS, and incubated with 500 μg/ml of anti-mouse IgM-AP (Southern Biotechnology) or anti-mouse IgA-AP (Life technologies) in a solution of 0.05% Tween-20 in PBS and 1% gelatin for 2 hours at 37°C. Plates were washed three times with 0.05% Tween-20 in PBS and developed for 18 hours at 4°C in substrate buffer pH 10.25 containing 1M 2-amino-2-methyl-1-propanol (AMP) (Sigma), 0.1% Triton-X405 (Sigma), 0.01g/ml of 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Sigma). Plates were washed in dH2O, and resulting spots produced by antibody-secreting cells were enumerated visually.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISAs were performed to measure serum and BALF anti-α-1,3-glucan-IgM, and -IgA antibodies along with total IgE and anti-Bla g 2 IgE in the serum and total IgE in the BALF. Anti-α-1,3-glucan antibody ELISAs were conducted using high-binding EIA/RIA plates (Costar) coated with 2 μg/mL purified α-1,3-glucan. After incubation with serum or BALF, antibody binding was detected with alkaline phosphatase-conjugated goat anti-mouse IgM, (Southern Biotechnology, Birmingham, AL), or -IgA (Life Technologies). Standard curves were prepared using known quantities of α-1,3-glucan-specific IgM (A16) or IgA (J558) antibodies purified as described in Antibody reagents and flow cytometry. Anti-Bla g 2-IgE was detected by coating plates with 2 μg/mL natural Bla g 2 (Indoor Biotechnologies), and antibody was detected using alkaline phosphatase-conjugated goat anti-mouse IgE (Southern Biotechnology Associates, AL). Total IgE levels were determined by coating plates with 2 μg/mL rat anti-mouse IgE (Southern Biotechnology), and standard curves were prepared with known concentrations of IgE antibodies (Southern Biotechnology). For all ELISA assays, p-Nitrophenyl Phosphate Substrate (Sigma) was added, and color development was detected with a SPECTROstar Omega Reader (BMG Labtech) at 405nm.
Flow cytometric or ELISPOT oligosaccharide inhibition assays
Cockroach particulates were incubated with of 500 ng A16 antibody with 5-100 μg of either nigerose (a disaccharide with α-1,3-glucan linkages) (Sigma), sucrose (a disaccharide of glucose and fructose) (Sigma), or PBS alone. A16 antibody binding to cockroach particles was detected by secondary goat-anti mouse-FITC antibodies by flow cytometry. Additionally, during the ELISPOT protocol, 5-100 μg of nigerose (Sigma) or sucrose (Sigma) in RPMI + 2% FBS or media alone were incubated with 4×105 cells from the spleen of an adult mouse that was immunized with MK7 as a neonate. Following development as described above, resulting antibody-secreting cells were enumerated visually.
Antibody reagents and flow cytometry
Hybridoma (A16) and plasmacytoma (J558) were grown in serum-free RPMI-1640 (Hybridoma SFM, Gibco). IgM antibodies were purified from supernatants on Sepharose-6B columns coupled with RS3.1 anti-mouse IgH6a monoclonal antibody, and IgA antibodies were purified from Sepharose 4B affinity columns coupled with rat monoclonal anti-mouse IgA (Clone 11-44). Cockroach particulates, Enterobacter vaccine preparations, and timothy grass pollen were stained with purified monoclonal anti-α-1,3-glucan antibody A16 (IgM), anti-β-1,3-glucan antibody 744 (IgM) or anti-hen egg lysozyme (HEL) isotype control antibody MD4 (IgM). Cockroach fecal pellets were stained with antibodies against Bla g 2 (IgG1) or isotype control Der p 1 (IgG1) from Indoor Biotechnologies. Primary antibody incubation was followed by secondary goat-anti mouse-FITC antibodies against respective isotypes (Southern Biotechnology, Life Technologies). Cells from the BALF, mediastinal lymph node and lungs were stained for flow cytometry with fluorochrome-conjugated anti-mouse antibodies specific for the following cell surface markers, transcription factors, and cytokines: CD3 (145-2C11), CD4 (GK1.5), CD5 (53-7.3), CD8 (53-6.7), CD11c (HL3), CD11b (M1/70), CD19 (1D3), CD25 (3C7 & PC61), CD44 (IM7), CD62L (MEL-14), CD86 (GL1), CD90.2 (53-2.1), CD117 (2B8), CD127 (SB/199), B220 (RA3-6B2), IgE (R35-72), SiglecF (E50-2440), Ly6G (IA8), Gr-1 (RB6-8C5), KLRG1 (2F1), ST2 (RMST2-2) TER-119, DX5, GATA3 (L50-823), Foxp3 (R16-715), T-bet (O4-46), IL-2 (JES6-5H4), IFN-γ (XMG1.2), TNF (MP6-XT22), IL-4 (11B11), IL-6 (MP5-20F3), and IL-13 (ebio13A) (BD Biosciences and Ebiosciences).
Cell identification and T cell stimulation
Leukocytes were identified using flow cytometry by detecting specific cell surface markers based on the following gating strategy (See Supplemental Figure 1): T cells (CD3+CD4+), alveolar macrophages (Siglec-F+CD11c+), eosinophils (Siglec-F+CD11c-), neutrophils (CD11b+Ly6G+), dendritic cells (CD11b-CD11c+), immature dendritic cells (CD11b+CD11c+), macrophages (CD11b+CD11c-Ly6G-), B cells (B220+), IgE-bound non-B cells (B220-IgE+), basophils (B220-IgE+Ckit-), and mast cells (B220-IgE+Ckit+). T cells (CD3+CD4+) were further characterized as CD44high (antigen experienced) or CD25+ (activated). ILC2s were identified by excluding cells expressing CD3, CD4, CD8, CD19, B220, CD11b, CD11c, F4/80, GR1, TER119, and DX5 and gating on those that expressed CD90.2, ST2, KLRG1, CD127, CD25, and CD117. To detect cytokine production by T cells, cell suspensions were first restimulated with 0.08 μM PMA, 1.34 μM ionomycin (eBioscience Cell Stimulation Cocktail) in the presence of 3.0 μg/mL Brefeldin A (eBioscience) for 6 hours. These cells were then fixed and permeabilized with BD Cellular Fixation/Permeabilization Kit (554714) according to manufacturer's instructions before staining with antibodies targeting: IL-2, IFN-γ, TNF, IL-4, IL-6, and IL-13 (BD Biosciences). To determine T cell commitment, CD3+CD4+ T cells were stained with isotype control antibody or antibodies directed against GATA3, Foxp3, or T-bet together in conjunction with reagents from the eBiosciences Foxp3 staining buffer kit (00-5523-00). All flow cytometry analyses were performed on a FACSCalibur (BD Biosciences) or LSR II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Histology and fluorescence staining
For paraffin-embedded sections, mouse lungs or Blatella germanica roaches (Carolina Biologics) were fixed in 4% PFA, dehydrated by sequentially increasing concentrations of ethanol, and after xylene incubation, embedded in paraffin (Leica EG1150H). Six-micron lung sections were cut (Leica RM2235), rehydrated, and stained with either H&E (Sigma), Periodic Acid-Schiff (Sigma) stain, or Masson's trichrome stain (Sigma) according to manufacturer's instructions before being dehydrated and mounted in a xylene-based mounting media (poly-mount xylene). Cockroach sections were deparaffinized in CitriSolv (Fisher) and subjected to heat-induced antigen retrieval in 10mM citrate buffer (pH 6). Slides were quenched with 3% hydrogen peroxide, blocked, then incubated with A16 (IgM) or isotype control antibody (MD4). Antibody binding was detected using horseradish peroxidase-conjugated anti-mouse IgM antibody (Southern Biotechnology) and slides were developed using diaminobenzidine peroxidase (Vector Labs). Cockroach sections were also counterstained with hematoxylin. The sagittal section of the German cockroach (Fig 1H) is a composite of four sequential images created with Adobe Photoshop. Intact cockroach fecal pellets were stained with monoclonal anti-Bla g 2 (IgG1; Indoor Biotechnologies) or A16 (IgM), followed by anti-mouse IgG-FITC (Southern Biotech) or anti-mouse IgM 647 (Life Technologies) then mounted with Fluoromount (Southern Biotech). For BALF stains, identical volumes of mouse BALF cell suspensions were cytocentrifuged (Shandon Elliott cytocentrifuge) onto glass slides at 1,000 rpm for 5 minutes before staining with Modified Wrights stain (Sigma). Sections were imaged with a Leica DMRB microscope equipped with Hamatsu C4742-95 and 3CCD color cameras and appropriate filter cubes. Images involving fluorescence were acquired using OpenLAB 3.1 software (Agilent Technologies) and bright-field images were acquired using Adobe Photoshop 7.0.
Figure 1. Purified monoclonal anti-α-1,3-glucan antibody, A16, reacts with epitopes distributed throughout German cockroach (Blattella germanica).
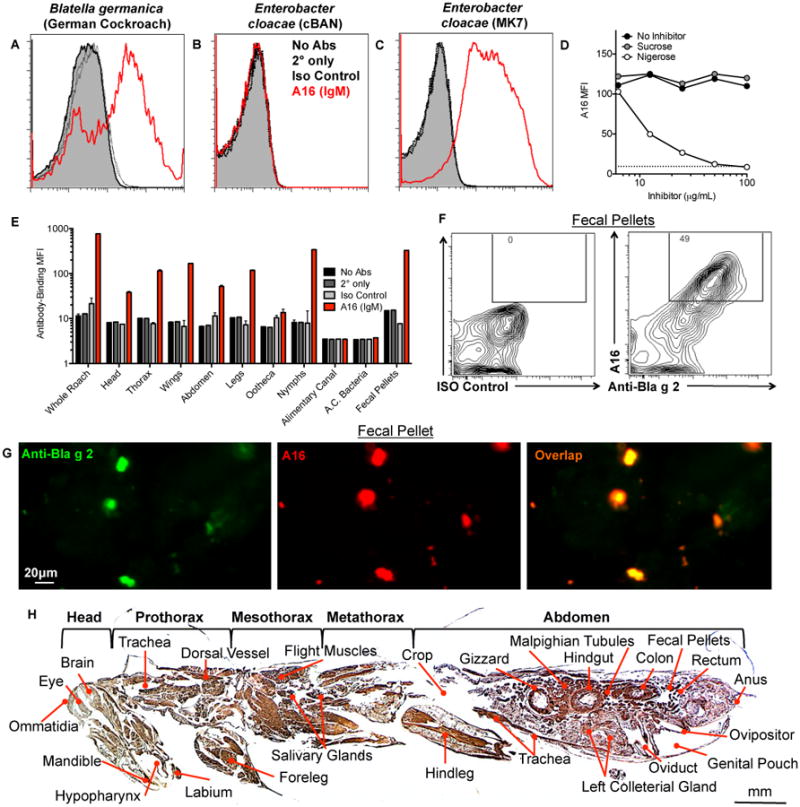
(A) German cockroach particles (Greer) or (B-C) Enterobacter cloacae vaccines (B) cBAN or (C) MK7 were stained with monoclonal anti-α-1,3-glucan IgM antibody, A16, or isotype control antibody and antibody binding was determined by flow cytometry. (D) Cockroach particles (dotted line) were incubated with A16 antibody or PBS alone (no inhibitor) in the presence of increasing concentrations of nigerose or control disaccharide sucrose, and the MFI of A16 binding to cockroach particles was determined. (E) Homogenized cockroach parts (Carolina Biologics) or fecal pellets, or gut bacteria were incubated with A16 antibody or isotype control antibody, and the MFI of A16 antibody binding was determined. (F, G) German cockroach fecal pellets were incubated with anti-Bla g 2 and A16 or isotype control antibodies and antibody binding was detected by (F) flow cytometry and (G) fluorescence microscopy. (H) Whole German cockroaches (Carolina Biologics) were embedded in paraffin and sagittal sections were subjected to antigen-retrieval prior to staining with A16 antibody (amber) and counterstaining with hematoxylin.
Single-frequency forced oscillation technique for mechanical ventilation
Mice were anesthetized with ketamine xylazine (100 mg/kg) and pancuronium bromide (0.8 mg/kg), then the tracheas was cannulated with an 18-G tube connected to the respiratory and expiratory ports of a Flexivent ventilator (SCIREQ), from which each mouse was ventilated at a rate of 160 breaths per minute. After acquiring baseline resistance measurements without challenge, increasing concentrations (10-50 mg/mL) of methacholine (Sigma) were vaporized, and total respiratory resistance (Rrs) was recorded every 12 seconds continuously for up to 3 minutes. Averages from each methacholine dose were calculated from 4-6 mice per group to determine airway hyperreactivity.
In vitro T cell priming assays
300,000 MedLN cells from each mouse that contained similar frequencies of T cells, B cells, and DCs as the other groups of mice were incubated with 5-50μg of cockroach particles (used for allergic challenge) in DMEM (Gibco) supplemented with 10% Fetal Bovine Serum (FBS), 100U/mL penicillin and streptomycin, 2.5μg/mL amphotericin, and 100uM 2ME for 5 days. The percentage of CD3+CD4+ T cells expressing CD25 were measured by flow cytometry.
Sample size and statistics
Values represent the mean ± SEM from 3 independent experiments with 5-10 mice per group. Statistical calculations described below were performed with Prism 4.0 software (GraphPad). Comparison of three or more groups was performed by a one-way ANOVA test followed by Tukey's post-hoc analysis and data from only two groups were analyzed by a two-tailed unpaired t test to determine statistical significance. In Figures 2-4, 6 and 7, and Supplemental Figures 2 and 3, only statistically significant differences between mice immunized with PBS or cBAN and differences between mice immunized with cBAN or MK7 are displayed. In Figure 5, differences between mice immunized with PBS and β-1,3-glucan and differences between mice immunized with β-1,3-glucan and α-1,3-glucan are displayed. In the figures, statistically significant differences are indicated as p-values of *<0.05, **<0.01, and ***<0.001.
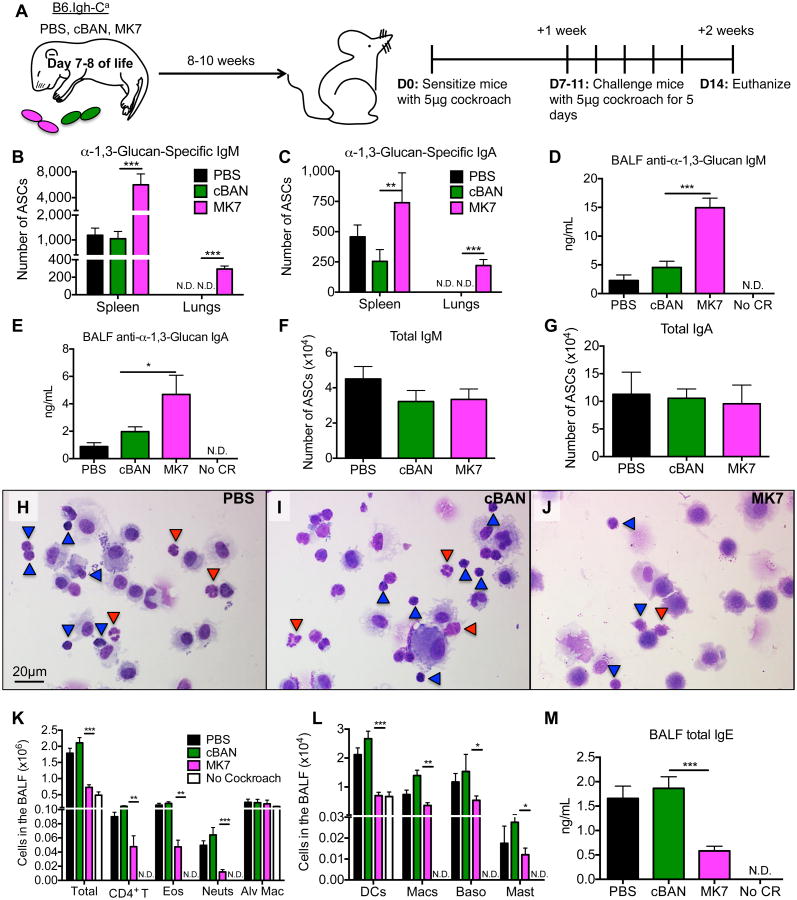
Figure 2. Following challenge with cockroach allergen, α-1,3-glucan-specific IgM- and IgA-secreting cells are detectable in the lungs of B6.Igh-Ca mice immunized with MK7 as neonates.
(A) Neonatal B6.Igh-Ca mice were immunized i.p. with 5×107 cBAN or MK7 or treated with PBS. At 8 to 10 weeks of age, these mice were sensitized with cockroach allergen, rested for seven days, challenged for 5 consecutive days, and euthanized two days later for analysis. (B-C) The number of α-1,3-glucan-specific IgM- and IgA- antibody secreting cells (ASCs) and (F, G) total IgM- and IgA-secreting cells in the spleen and lungs were determined by ELISPOT. (D, E) Levels of anti-α-1,3-glucan-IgM and -IgA antibodies in the BALF were determined by ELISA. (H-J) Identical volumes of BALF were cytocentrifuged and cells were stained with Modified Wrights Stain and lymphocytes (blue arrows) and neutrophils (red arrows) are indicated. (K-L) Cells in the BALF were identified by flow cytometry as documented in Supplemental Figure 1 and quantified. (M) Total IgE in BALF of indicated mice was measured by ELISA. Values represent the mean ± SEM from 3 independent experiments with 5-10 mice per group, and values that were not detectable are noted as “N.D.” Data were analyzed by ANOVA, and statistically significant differences are indicated as *p<0.05, **p<0.01, and ***p<0.001.
Figure 4. MedLNs of mice immunized with MK7 as neonates contain decreased numbers of TH2-committed and cytokine-producing CD4+ T cells following cockroach allergy induction.
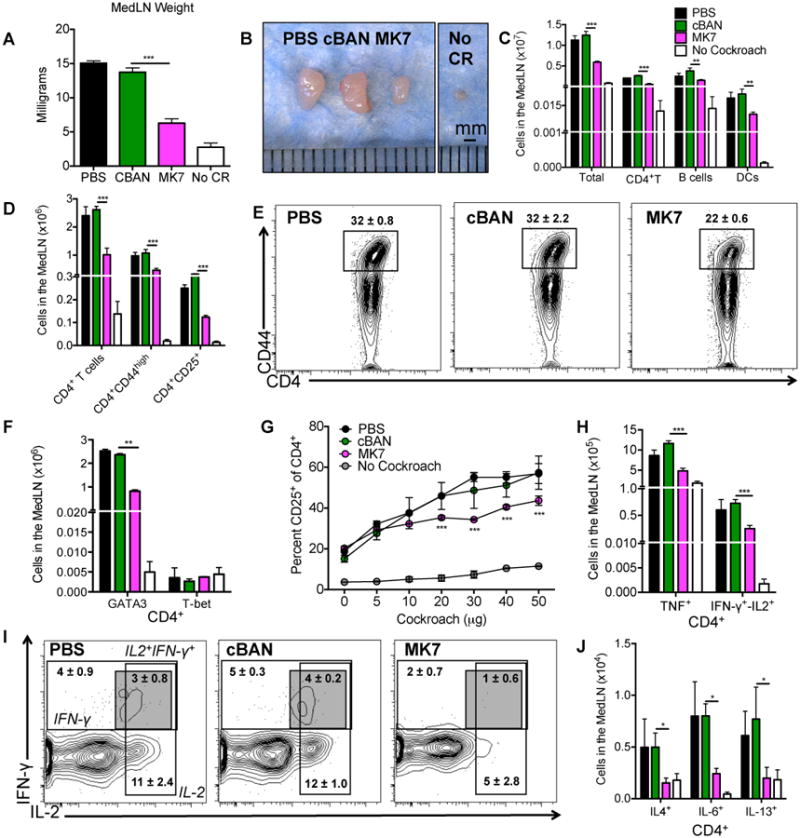
(A-C) Total weight, representative images, and the cellular composition of MedLNs harvested from B6.Igh-Ca mice are displayed following sensitization and challenge with cockroach allergen. (D) The number of CD4+ T cells that are CD44high (antigen experienced) or CD25+ (activated) were enumerated from the MedLN and (E) CD4+ T cells that are CD44high are displayed. (F) The frequency of cells from the MedLN that are CD4+ and GATA-3+ or T-bet+ was determined. (G) 300,000 MedLN cells from each mouse were incubated with 5-50μg of cockroach allergen and the percent of CD4+ T cells expressing CD25 was determined after 5 days. (H-J) MedLN cells were stimulated with PMA and ionomycin in the presence of Brefeldin A and CD4+ T cells were assayed for production of (H, I) TH1-associated cytokines (TNF or IFN-γ/IL-2) or (J) TH2-associated cytokines (IL-4, IL-6, and IL-13) by intracellular staining and flow cytometry as documented in Supplemental Figure 1. CD4+ T cells producing IFN-γ, IL-2, or IFN-γ and IL-2 are displayed along with mean ± SEM. (I) Values represent the mean ± SEM from 3 independent experiments with 5-10 mice per group. Data were analyzed by ANOVA, and statistically significant differences are indicated as *p<0.05, **p<0.01, and ***p<0.001.
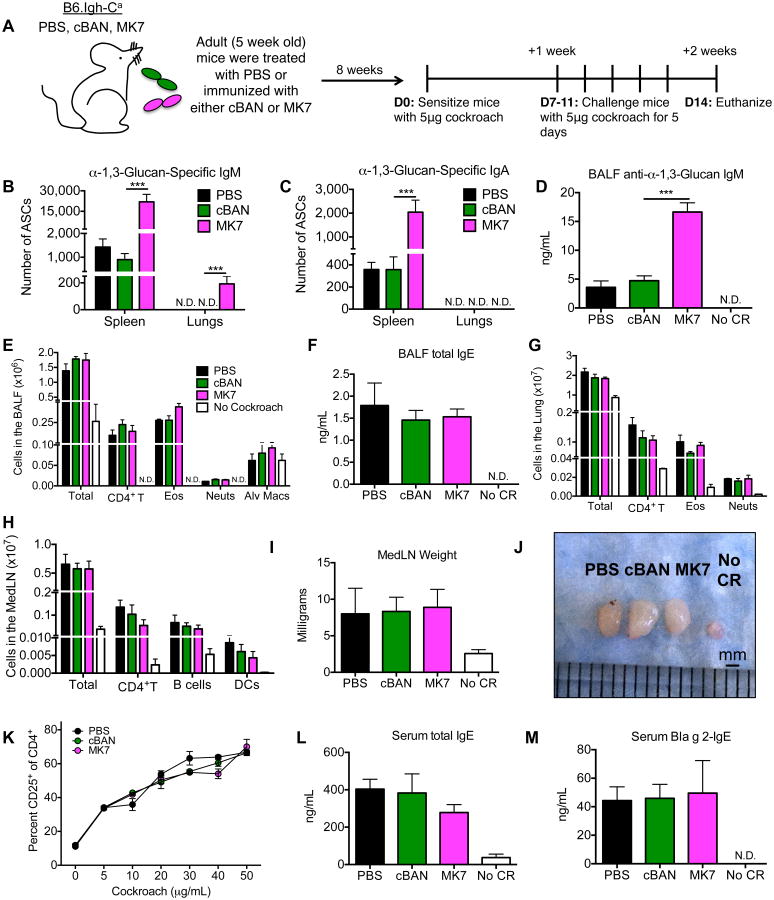
Figure 6. Immunization of adult B6.Igh-Ca mice with MK7 has no effect on the development of cockroach allergy.
(A) Five-week-old B6.Igh-Ca mice were immunized i.p. with 5×107 cBAN or MK7 or administered PBS. These mice were rested for eight weeks and were then sensitized with cockroach allergen, rested for seven days, challenged for 5 consecutive days, and euthanized two days later for analysis. (B, C) The number of α-1,3-glucan-specific IgM- and IgA-secreting cells isolated from the spleen and lungs were quantified by ELISPOT (D) and titers of anti-α-1,3-glucan IgM and IgA antibodies from the BALF were determined by ELISA. (E, G) Cells from the bronchoalveolar space and pulmonary parenchyma were identified by flow cytometry as documented in Supplemental Figure 1 and quantified. (F) BALF total IgE was measured by ELISA. (H-J) MedLNs were photographed and weighed, and the numbers of CD4+ T cells, B cells, and DCs in the LN were quantified. (K) 300,000 MedLN cells were incubated with 5-50μg of cockroach allergen for five days and the percent of CD4+ T cells expressing CD25 was determined. (L, M) The amount of serum total IgE and anti-Bla g 2-IgE measured by ELISA. Values represent the mean ± SEM from 3 independent experiments with 5-10 mice per group, and values that were not detectable are noted as “N.D.” Data were analyzed by ANOVA, and statistically significant differences are indicated as *p<0.05, **p<0.01, and ***p<0.001.
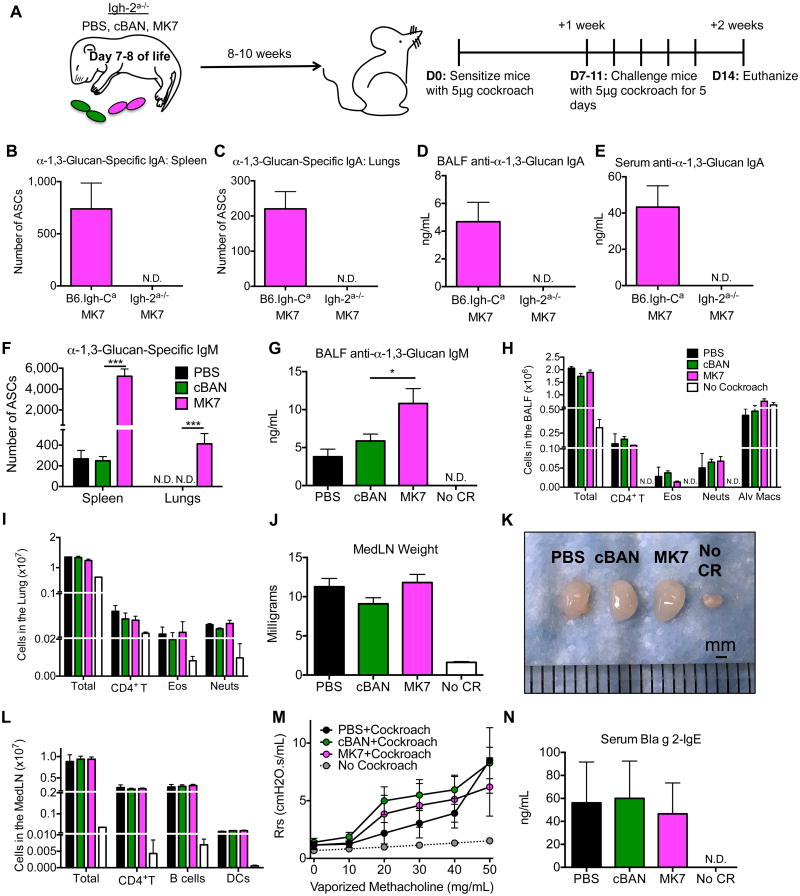
Figure 7. Neonatal immunization of mice that do not make IgA antibodies to α-1,3-glucan with MK7 does not attenuate cockroach-induced allergic disease.
(A) C57BL/6 (Igh-Cb Igh-2+/+) mice were crossed to IgA-deficient mice (Igh-Ca Igh-2-) to generate mice that do not make IgA antibodies to α-1,3-glucan (Igh-2a-/-). Neonatal Igh-2a-/- mice were immunized i.p with 5×107 of cBAN or MK7 or treated with PBS. At 8 to 10 weeks of age, these mice were sensitized and challenged i.t. with cockroach allergen. (B-C, F) The number of α-1,3-glucan-specific IgA- and IgM-secreting cells from the (B, F) spleen and (C, F) lungs was determined by ELISPOT and (D-E, G) levels of anti-α-1,3 glucan-specific IgA and IgM from the (D, G) BALF and (E) serum was determined by ELISA. (H, I) CD4+ T cells, eosinophils, and neutrophils in the BALF and enzymatically-digested pulmonary parenchyma were identified as documented in Supplemental Figure 1 and quantified. (J-L) MedLNs were photographed, weighed, and the number of CD4+ T cells, B cells, and DCs of MedLN isolated from these mice was determined. (M) Respiratory system resistance (Rrs) was calculated from mice that were mechanically ventilated and challenged with the indicated concentrations of vaporized methacholine. (N) The amount of anti-Bla g 2-IgE in the serum of the indicated mice was determined by ELISA. Values represent the mean ± SEM from 3 independent experiments with 5-10 mice per group, and values that were not detectable are noted as “N.D.” Data were analyzed by ANOVA, and statistically significant differences are indicated as *p<0.05, **p<0.01, and ***p<0.001.
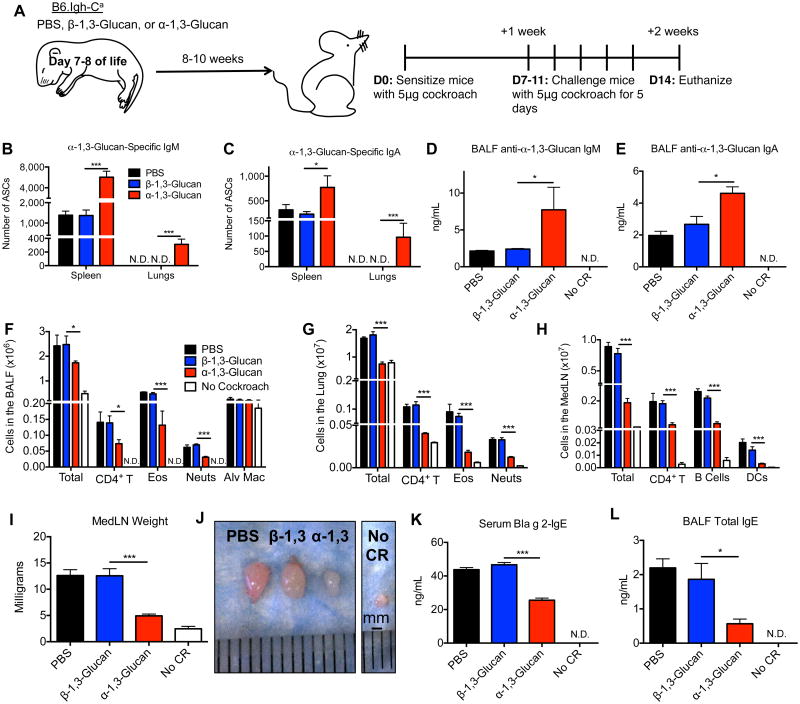
Figure 5. Neonatal immunization with purified α-1,3-glucan results in the localization of α-1,3-glucan-specific IgM- and IgA-secreting cells in the lung and protection against cockroach allergy.
(A) Neonatal B6.Igh-Ca mice were immunized i.p. with 25μg β-1,3-glucan or α-1,3-glucan or treated with PBS. At 8 to 10 weeks of age, these mice were sensitized and challenged i.t. with cockroach allergen. (B, C) The number of α-1,3-glucan-specific IgM- and IgA- secreting cells from the lungs and spleen were enumerated and (D, E) anti-α-1,3-glucan-IgM and -IgA from the BALF was quantitated by ELISA. (F-G) Cells were identified from the BALF and enzymatically-digested pulmonary parenchyma as documented in Supplemental Figure 1 and enumerated. (H-J) MedLNs were photographed, weighed, and CD4+ T cells, B cells, and DCs were quantified. (K, L) ELISA was used to determine the amount of serum anti-Bla g 2-IgE and BALF total IgE. Values represent the mean ± SEM from 2 independent experiments with 5-10 mice per group, and values that were not detectable are noted as “N.D.” Data were analyzed by ANOVA, and statistically significant differences are indicated as *p<0.05, **p<0.01, and ***p<0.001.
Results
Cockroach allergen expresses α-1,3-glucan epitopes
Having demonstrated that environmental allergens, such as A. fumigatus and HDM, display antibody-reactive epitopes associated with certain bacteria (15, 16), we expanded our studies to analyze German cockroach (Blattella germanica) allergen. Cockroach particles were bound by purified monoclonal anti-α-1,3-glucan antibody, A16 (IgM,λ1) (Figure 1A). We have previously demonstrated that some strains of Enterobacter, such as MK7, express α-1,3-glucan (Figure 1C) but other strains of Enterobacter such as cBAN do not (Figure 1B) (10, 33, 41). Binding of monoclonal antibody A16 to cockroach allergen is inhibited by nigerose, a disaccharide with α-1,3-glucan glycosidic linkages, but not by sucrose (Figure 1D). Staining of dissected (Figure 1E) and paraffin-embedded histologic sections of German cockroach (Figure 1H) revealed that A16 antibody, but not an isotype control antibody, binds many external and internal structural components of the insect, including cockroach allergen Bla g 2-expressing fecal pellets (Figure 1E-H). A16 antibody does not bind components of the alimentary canal (A.C), its contents, bacteria isolated from the A.C, (Figure 1E) or the crop of the insect (Figure 1H). Thus, the α-1,3-glucan epitope is widely expressed on German cockroaches, including Bla g 2-expressing fecal pellets.
Immunization of neonatal B6.Igh-Ca mice with α-1,3-glucan-bearing Enterobacter (MK7) results in the accumulation of α-1,3-glucan-specific IgM- and IgA-secreting cells in the lung following sensitization with cockroach allergen
In order to focus our studies on the protective effects of α-1,3-glucan-specific B cells and antibodies on the development of cockroach allergy, we used congenic C57BL/6 mice expressing the Igh-Ca haplotype (B6.Igh-Ca), which unlike the background C57BL/6 strain, express a J558 immunoglobulin variable heavy chain (IgVH) gene segment that enables them to respond α-1,3-glucan and produce anti-α-1,3-glucan antibodies. As a model for early exposure to microbial α-1,3-glucan, neonatal (7- to 8-day old) B6.Igh-Ca mice were immunized i.p. with Enterobacter vaccine, MK7. Control littermate mice were immunized with Enterobacter vaccine cBAN or treated with PBS, and all groups were rested until they were 8- to 10-weeks of age (Figure 2A). At this time, these groups of mice were sensitized with cockroach allergen i.t., rested for seven days, then challenged for 5 consecutive days with cockroach allergen and subsequently euthanized two days later for analysis (Figure 2A).
Compared to controls, mice immunized with MK7 have significantly increased numbers of α-1,3-glucan-specific IgM- and IgA-secreting cells in the spleen (Figure 2B, C) and bone marrow as well as higher levels of serum anti-α-1,3-glucan antibodies (unpublished observations). ELISPOTs from splenic α-1,3-glucan-specific IgM-secreting cells were inhibited by nigerose, a disaccharide with α-1,3-glucan linkages, but not sucrose (unpublished observations). α-1,3-glucan-specific antibody-secreting cells were not detectable in the lungs of control or mice immunized as neonates prior to sensitization with cockroach allergen (unpublished observations). However, following sensitization and challenge with cockroach allergen, only mice immunized with MK7 have detectable frequencies of α-1,3-glucan-specific IgM- and IgA-secreting cells in the lung tissue (Figure 2B, C) and increased levels of corresponding antibodies in the BALF (Figure 2D, E) compared to control mice. The increased frequency of pulmonary α-1,3-glucan-specific antibody-secreting cells (ASCs) is not associated with an overall increase in total numbers of pulmonary IgM- or IgA-secreting cells (Figure 2F, G). Thus, immunization of neonatal mice with α-1,3-glucan-bearing MK7 and subsequent challenge of these mice with cockroach allergen as adults results in an increased frequency of α-1,3-glucan-specific IgM- and IgA-secreting cells in the lung tissue and antigen-specific antibody in the BALF compared to control mice.
Neonatal immunization with α-1,3-glucan-bearing Enterobacter (MK7) suppresses cockroach-induced allergic responses in the lung
Given the reactivity of anti-α-1,3-glucan antibodies with cockroach allergens, we asked if increasing the number of α-1,3-glucan-specific B cells in the adult by neonatal immunization would affect the response to cockroach allergen challenge during adult life. Staining of cytocentrifuged cells from the BALF with Modified Wright's stain revealed a decreased number of neutrophils (red arrows) and lymphocytes (blue arrows) from mice treated with MK7 as neonates compared to control mice (Figure 2H-J). Additionally, mice immunized with MK7 as neonates had lower numbers of CD4+ T cells, eosinophils, neutrophils, antigen-presenting cells, basophils, and mast cells in the BALF (Figure 2K, L) and pulmonary parenchyma along with ILCs in their lungs (Figure 3D, E) compared to control mice. Pulmonary DCs, immature DCs, parenchymal macrophages, and alveolar macrophages from mice immunized with MK7 as neonates express decreased amounts of the MHC II costimulatory molecule, CD86 (Figure 3F) compared to control mice. Neonatal immunization with MK7 is associated with reduced total IgE in the BALF (Figure 2M) and serum (Figure 3J) as well as decreased levels of serum anti-Bla g 2-IgE (Figure 3K) compared to control mice.
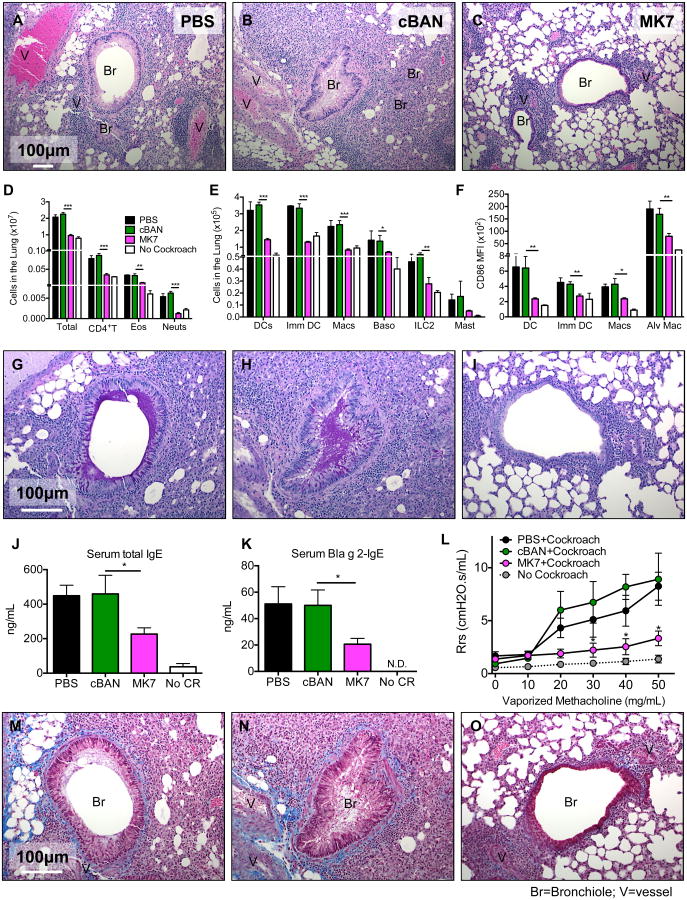
Figure 3. B6.Igh-Ca mice immunized with MK7 as neonates exhibit suppressed development of pulmonary airway disease.
(A-C) Neonatal B6.Igh-Ca mice were immunized with cBAN or MK7 or treated with PBS, and at 8 to 10 weeks of age, these animals were sensitized and challenged with cockroach allergen. Paraffin-embedded lungs were stained with (A-C) Hematoxylin and Eosin, (G-I) Periodic Acid-Schiff, (M-O) or Masson's Trichrome stains. (D, E) Cells from enzymatically digested lungs of the indicated mice were identified by flow cytometry as documented in Supplemental Figure 1 and quantified. (F) CD86 MFI on the indicated APC populations from the lung digest was determined. (J, K) Levels of total IgE and anti-Bla g 2-IgE in the sera of indicated groups of mice was measured by ELISA. (L) Respiratory system resistance (Rrs) was calculated from mice that were mechanically ventilated and challenged with the indicated concentrations of vaporized methacholine. Values represent the mean ± SEM from 3 independent experiments with 5-10 mice per group. Values that were not detectable are noted as “N.D.” Data were analyzed by ANOVA, and statistically significant differences are indicated as *p<0.05, **p<0.01, and ***p<0.001.
The lungs of unchallenged mice have minimal cellular infiltration around the bronchioles and vessels (Supplemental Figure 1Q), an absence of mucin-producing cells in the bronchus (Supplemental Figure 1R), and no evidence of excessive collagen deposition around the vessels and bronchi (Supplemental Figure 1S). Following sensitization and challenge with cockroach allergen as adults, mice treated with PBS or cBAN as neonates exhibit extensive cellular infiltration around the bronchioles, pulmonary vessels, and the smaller alveolar airways (Figure 3A, B). Epithelial cells within the bronchioles of these animals are hypertrophic and numerous bronchioles are obstructed by mucin plugs (Figure 3G-H magenta). Collagen (blue) accumulates around the airways and in the alveolar spaces (Figure 3M-N), and these mice exhibit impaired lung function, as indicated by the increase in respiratory system resistance (Rrs) following challenge with vaporized methacholine (Figure 3L). In contrast, mice immunized with MK7 as neonates have (i) markedly reduced cellular infiltrates around bronchioles and vessels (Figure 3C), (ii) lack of mucin-producing cells and mucin plugs in the bronchioles (Figure 3I), (iv) decreased collagen deposition surrounding the bronchioles and alveolar spaces (Figure 3O), and (iv) Rrs comparable to normal mice after challenge with vaporized methacholine (Figure 3L). Collectively these results demonstrate that neonatal immunization with α-1,3-glucan-bearing MK7 decreases the infiltration of allergy-associated cells in to the bronchoalveolar space and lungs along with the production of IgE and dramatically affects airway pathology and pulmonary function resulting from sensitization and challenge with cockroach allergen.
MedLNs of mice immunized with α-1,3-glucan-bearing Enterobacter (MK7) as neonates contain decreased numbers of TH2-committed and cytokine-producing CD4+ T cells following cockroach allergy induction
Priming and activation of allergen-specific memory TH2 cells in the lung-draining lymph node (42) is responsible for driving and maintaining the pathogenicity associated with allergic disease (43). MedLNs isolated from mice immunized with MK7 as neonates and challenged with cockroach as adults weigh less (Figure 4A), are smaller (Figure 4B), and display reduced cellularity (Figure 4C) compared to control mice. Mice immunized with MK7 as neonates have decreased numbers of CD4+ T cells in their MedLN that are CD44high, CD25+, or GATA-3+, but there is no difference in the number of CD4+T-bet expressing cells compared to control mice (Figure 4D-F). MedLN cells from each mouse that contained similar numbers of CD4+ T cells, DCs, and B cells as mice from the other groups were incubated with cockroach allergen in vitro for 5 days to evaluate the ability of T cells to become activated. Mice immunized with MK7 as neonates have a lower percent of CD4+ T cells that expressed CD25 in response to antigen stimulation compared to control mice (Figure 4G). The number of CD4+T cells from the MedLN that produce TH1-associated cytokines (TNF or IFN-γ/IL-2) or TH2-associated cytokines (IL-4-, IL-6- and IL-13) following stimulation is also decreased in mice immunized with MK7 compared to the control group (Figure 4H-J). Overall, mice immunized with MK7 as neonates have MedLNs containing fewerCD4+ T cells that are CD44high, TH2-committed, cytokine-producing, and responsive to cockroach stimulation compared to control mice.
Neonatal immunization with purified α-1,3-glucan, but not β-1,3-glucan, suppresses cockroach allergy
To determine if the decreased allergic response to cockroach particulates following neonatal immunization with MK7, compared to cBAN, is due expression of the α-1,3-glucan epitope by MK7, we treated neonatal littermate B6.Igh-Ca mice with PBS or immunized them with purified α-1,3-glucan or purified β-1,3-glucan as a control (Figure 5A). Devoid reactivity of anti-β-1,3-glucan monoclonal antibody 744 (IgM,κ) with cockroach particulates (unpublished observations) indicates an absence of β-1,3-glucan epitopes on cockroach allergen. At 8- to 10-weeks of age, immunized mice were sensitized i.t. with cockroach allergen, and challenged as described in Figure 5A. Similar to mice immunized with MK7 as neonates, there were increased numbers of α-1,3-glucan-specific IgM- and IgA-secreting cells in the spleens of mice treated with α-1,3-glucan as neonates (Figure 5B, C) and there were no detectableα-1,3-glucan-specific antibody-secreting cells in the lungs of adult mice treated with either glucan as neonates prior to sensitization (not shown). However, following sensitization and challenge with cockroach allergen, α-1,3-glucan-specific IgM- and IgA-secreting cells are detectable in the lungs (Figure 5B, C) along with increased titers of anti-α-1,3-glucan IgM and IgA in the BALF (Figure 5D, E). Mice immunized with purified α-1,3-glucan as neonates exhibit suppressed development of cockroach allergy, as evidenced by decreased infiltration of CD4+ T cells, eosinophils, and neutrophils in the BALF (Figure 5F) and lungs (Figure 5G), decreased cellularity of the MedLN (Figure 5H-J), reduced titers of serum anti-Bla g 2 IgE (Figure 5K), and lower total IgE in the BALF (Figure 5L) compared to PBS treated or β-1,3-glucan-immunized mice. Thus, similar to immunization with MK7 bacteria during neonatal life, early life immunization with purified α-1,3-glucan, but not β-1,3-glucan, results in suppression of cockroach allergy following sensitization and challenge to cockroach allergen. As further evidence for the specificity of the α-1,3-glucan antibody response's contribution to the protection against allergic disease, MK7 immunization of C57BL/6 mice (Igh-Cb), which make poor antibody responses to α-1,3-glucan, does not result in protection against the development of cockroach allergy (Supplemental Figure 2).
Immunization of adult B6.Igh-Ca mice with α-1,3-glucan-expressing Enterobacter (MK7) does not effect the development of cockroach allergy
Because adult mice mount a robust antibody response to α-1,3-glucan (41), we next determined if immunization of adult mice with MK7 would protect against the development of allergic disease in response to cockroach allergen. Five-week old adult littermate B6.Igh-Ca mice were treated with PBS or immunized i.p. with cBAN or MK7, and similar to the mice immunized as neonates, these mice were rested for 8 weeks before being sensitized and challenged with the allergen, as described in Figure 6A. Adult B6.Igh-Ca mice immunized with MK7 have increased numbers of α-1,3-glucan-specific IgM and IgA-secreting cells in the spleen (Figure 6B, C) and bone marrow and increased titers of circulating anti-α-1,3-glucan antibodies in sera (unpublished observations) compared to control mice. Following sensitization and challenge with cockroach allergen, only α-1,3-glucan-specific IgM-secreting cells (Figure 6B) and anti-α-1,3-glucan IgM antibody (Figure 6D), but no IgA-secreting cells or respective antibodies (Figure 6C), were detected in the lungs of mice treated with MK7 as adults. Compared to adult mice treated with PBS or cBAN, adult mice immunized with MK7 demonstrate no differences in the number of allergy-associated cells in the BALF (Figure 6E) or lungs (Figure 6G), cellular composition of the MedLN (Figure 6H-J), activation of CD4+ T cells following stimulation with cockroach allergen (Figure 6K), total IgE in the BALF (Figure 6F) or serum (Figure 6L), or anti-Bla g 2-IgE in the serum (Figure 6M). Therefore, unlike neonatal immunization with MK7, adult immunization with MK7 does not result in allergic suppression. The protective effect of neonatal vaccination is not due to an overall increase in the frequency of regulatory T cells or TH1 cells, as the numbers of these cells are similar in mice immunized as neonates or adults with MK7 (unpublished observations). Instead, neonatal immunization with MK7 results in a unique population of α-1,3-glucan-specific IgA-secreting cells in the lungs following sensitization and challenge with cockroach allergen (Figure 2C) that is not detectable in the lungs of mice immunized with MK7 as adults (Figure 6C).
Immunization of neonatal mice that cannot make IgA antibodies to α-1,3-glucan with MK7 does not result in suppressed cockroach allergy
To determine if anti-α-1,3-glucan IgA is necessary to protect against cockroach-induced allergic disease, we crossed C57BL/6 mice (Igh-Cb) with IgA-deficient mice (Igh-2-/-) that expressed the Igh-Ca haplotype. The F1 generation of these mice simultaneously express paternally derived Igh-Cb haplotype-expressing B cells that can make IgA (Igh-2+/+) but cannot make antibodies to α-1,3-glucan, as well as maternally derived Igh-Ca haplotype expressing B cells that can make anti-α-1,3-glucan IgM, but not anti-α-1,3-glucan IgA. Therefore, these F1 mice (Igh-2a-/-) are specifically deficient in their ability to generate an IgA antibody response to α-1,3-glucan. Neonatal Igh-2a-/- littermate pups were immunized with cBAN or MK7 or treated with PBS. At 8- to 10-weeks of age, these animals were sensitized and challenged with cockroach allergen as described above (Figure 7A). As expected, IgA-secreting cells and IgA antibodies, but not α-1,3-glucan-specific IgA-secreting cells or specific antibodies, were detectable in the serum and multiple tissues analyzed from these mice (Figure 7B-E). Igh-2a-/- mice immunized with MK7 as neonates have increased numbers of α-1,3-glucan-specific IgM-secreting cells in the spleen (Figure 7F) and bone marrow and anti-α-1,3-glucan IgM antibodies in the serum, and there were no detectable α-1,3-glucan-specific antibody-secreting cells in the lungs of adult mice prior to allergen sensitization (not shown). Sensitization and challenge with cockroach allergen of Igh-2a-/- mice immunized with Enterobacter MK7 as neonates results in the localization of α-1,3-specific IgM-secreting cells in the lungs (Figure 7F) and increased levels of anti-α-1,3-glucan IgM antibody in the BALF (Figure 7G) compared to control mice. The cellularity of the BALF (Figure 7H), lungs (Figure 7I), and MedLN (Figure 7J-L) as well as airway hyperresponsiveness (Figure 7M) and Bla g 2-IgE levels in the serum (Figure 7N) is similar within these groups regardless of neonatal immunizations. These results demonstrate that the protective effects of neonatal immunization with MK7 against the development of cockroach allergy are dependent on the IgA antibody response to α-1,3-glucan.
Discussion
In this study, we demonstrate that neonatal immunization with purified α-1,3-glucan or an α-1,3-glucan-expressing bacterial vaccine derived from the Enterobacter MK7 provides significant protection against the development of cockroach allergy. This protection is the result of a specific response to the α-1,3-glucan moiety on the Enterobacter, as neither immunization of B6.Igh-Ca mice with β-1,3-glucan or the α-1,3-glucan-deficient Enterobacter strain cBAN nor immunization of poorly-responding C57BL/6 mice with MK7 has an effect on the allergic response to cockroach particles. Additionally, the expression of the α-1,3-glucan epitope on the allergen is critical for this immune protection, as sensitization and challenge with α-1,3-glucan-free timothy grass pollen did not result in the localization of α-1,3-glucan-specific IgA-secreting B cells in the lung, and mice immunized with MK7 as neonates did not exhibit suppressed development of timothy grass pollen-induced allergic disease (Supplemental Figure 3). Despite a robust antibody response, B6.Igh-Ca mice immunized with MK7 as adults are not protected against allergic disease. These results are similar to our findings in the A. fumigatus fungal and HDM allergy models (15, 16), in which there was a striking age-related dependence for vaccination to induce effective protection against allergic reactions. A similar age-related effect has also been shown to be critical for the induction of iBALT (44). Suppressed development of cockroach-associated allergic responses following neonatal immunization with MK7 is a result of α-1,3-glucan-specific IgA-secreting cells, which localize to the lung following allergen sensitization and challenge with the allergens. Mice deficient in their ability to make anti-α-1,3-glucan IgA that are immunized with MK7 as neonates and B6.Igh-Ca mice immunized with MK7 as adults do not exhibit protection from cockroach induced allergic disease, despite a robust α-1,3-glucan specific IgM response.
In previous studies we detected α-1,3-glucan-specific splenic B cell clonal precursors during neonatal life (5 to 11 days of life) that have the potential to give rise to progeny that secrete predominantly α-1,3-glucan-specific IgM and IgA in culture supernatants. With age (between day 12 to 30 of life), the overall number of B cells secreting anti-α-1,3-glucan IgM remains constant, however the number of cultures containing both IgM and IgA or only IgA decreases to a much lower frequency (10). A possible reason for this shift is that in the developing neonate, there is a higher abundance of B1 cells in the spleen compared to adult mice (12), and B1 cells switch more readily to IgA than other B cell subsets (14, 45). Therefore, neonatal exposure to α-1,3-glucan-bearing Enterobacter may select a subset of early CD5+ B cells with a higher intrinsic potential for anti-α-1,3-glucan IgA secretion into the adult repertoire, or in to mucosal sites, which would account for the protective effects of neonatal immunization with MK7.
Our previous studies demonstrated that IgM antibodies stimulated by microbes (Streptococcus pyogenes and Streptococcus pneumoniae) during early life are protective against the development of A. fumigatus and HDM allergy during adult life (15, 16). However, the studies presented in this body of work clearly demonstrate that anti-α-1,3-glucan IgA, but not IgM, is protective against the development of cockroach allergy. Protection mediated by IgA, but not IgM, may be the net result of several factors. One distinct difference between this model and the two published studies is that immunization of neonates with PC-expressing S. pneumoniae does not stimulate anti-PC IgA antibodies (16), however, immunization of neonatal and adult mice with α-1,3-glucan-expressing Enterobacter stimulates a robust anti-α-1,3-glucan IgA antibody response (37). Since TLR stimulation is a signal that can promote T-independent class switching to IgA (46), it is possible that IgA antibodies are uniquely induced in this model by Gram-negative Enterobacter, but are not induced by Gram-positive streptococci. α-1,3-glucan-specific IgM secreting cells selected into the adult repertoire as a result of neonatal immunization with MK7 express antibodies with the M104E idiotype (10), which has 100 fold lower affinity for α-1,3-glucan compared to α-1,3-glucan-specific B cells that express the J558 idiotype (9). Additionally, terminal deoxynucleotidyl transferase (TDT) is necessary for the formation of high affinity J558-idiotype expressing antibodies to α-1,3-glucan, and expression of TDT is low during neonatal life (47). Therefore α-1,3-glucan-specific IgM antibodies stimulated by MK7 during early life express relatively low-affinity BCRs and antibodies. Since α-1,3-glucan can be expressed by enteric commensal organisms (33) it is possible that the α-1,3-glucan-specific IgA-secreting cells that localize to the lung following cockroach sensitization express antibodies that are more somatically hypermutated and thus may have higher affinity for α-1,3-glucan compared to the anti-α-1,3-glucan IgM antibodies. Last, although we know α-1,3-glucan epitopes are expressed in multiple sites in the German cockroach, we do not know how the glycan is arrayed on the allergenic particles. The α-1,3-glucan epitope may be linear or branched and could exist as an oligomer or a polymer (48, 49). M104E antibodies preferentially bind oligos that are no larger than trisaccharides, whereas the higher affinity J558 antibody binds glycans arrayed as penta- and hexasaccharide (49). Depending on the way α-1,3-glucan epitopes are arrayed on cockroach particles it is possible that the allergen could be better recognized by IgA antibodies but not IgM antibodies. Therefore, antibody affinity and glycan presentation on the allergenic particles may be contributing factors to IgA-, but not IgM-, mediated protection against cockroach allergy.
IgA-mediated neutralization of allergens, bacterial pathogens, and viral particles associated with the mucosa is critical for immune protection (50). Selective IgA deficient patients have higher rates of atopic disorders than those without the deficiency (51, 52). In our model, anti-α-1,3-glucan IgA may be specially important in preventing the interaction of cockroach-derived Bla g 2/α-1,3-glucan complexes with innate receptors on DCs and epithelial cells in the lung, thus decreasing their activation of ILC2s and priming of TH2 cells. IgA antibody binding to pathogens or allergenic particulates may also promote more efficient mucosal clearance via the mucociliary escalator. In line with our studies, it was reported that intranasal administration of an anti-human IgA antibody to a ragweed pollen antigen (Amb a 1) within a few hours of allergen challenge significantly decreased the development of airway hyperreactivity, production of allergen-specific IgE, and secretion of TH2-associated cytokines in mice. This study also demonstrated that antibody-mediated protection was antigen dependent, as the anti-human Amb a 1 antibody did not protect in a model of ovalbumin challenge (53). Another study showed that DCs primed in the presence of cholera toxin B (CTB) and OVA stimulated the production of mucosal IgA from MedLN B cells, which suppressed the development of allergic disease. The authors suggested that CTB exposure suppressed allergic inflammation via secretory IgA, because the above phenotype was lost in pIgR-/- mice (54). However, IgM is also transported to the mucosa via pIgR and it is possible that the immunosuppressive effects of CTB may have been due to pulmonary mucosa-associated IgM and/or IgA.
Because α-1,3-glucan epitopes are expressed on gut microflora, fungi, and various oral biofilm-forming organisms it is likely that children can respond to these epitopes via mucosal (gut, pulmonary, or oral) immune responses during early life. Sampling of a cohort of 150 Caucasian and African American pediatric patients ranging from ages 1-15 years demonstrated that all children have detectable, but variable, levels of serum anti-α-1,3-glucan IgM antibodies irrespective of their asthma status (unpublished observations). There is currently no cure for asthma, and therapeutics for atopic diseases focus on treatment of symptoms. Individuals typified by long-term steroid or antihistamine use or those administered monoclonal antibodies against TH2-associated cytokines or cytokine receptors can develop resistance to these treatments over time (55, 56). Based on our understanding of the neonatal mouse B cell response to α-1,3-glucan, we suggest that therapeutic prophylaxis in the form of a vaccine or probiotic containing α-1,3-glucans during early life could be used to modulate neonatal B cells with the potential for long-lived suppression of asthma development to cockroach sensitization.
Supplementary Material
Acknowledgments
Thank you to: Drs. Denise Kaminski and Peter Burrows for critical reading of this manuscript. Dr. Jeffrey Sides for maintaining and dissecting the German cockroaches as well as assistance with identification of invertebrate anatomy along with Dr. James McClintock and Roy Kucuk at UAB, Dr Donald Mullins at Virginia Tech, Dr. James Nardi University of Illinois at Urbana-Champaign, and Dr. Coby Schal at NC State University. Drs. Andrew Macpherson for providing Enterobacter strain cBAN, Marta Feldmesser for antibodies against β-1,3-glucan, and Mary Anne Hamilton and Marissa Menard for special tissue processing, the UAB Neuroscience Molecular Detection core (P30 NS47466), and the UAB Program in Protease and Matrix Biology for assistance with the AHR measurements.
Footnotes
This work was supported by the National Institutes of Health (NIH) Grants AI14782-37 and AI100005-05 and T32 AI00705 as well as the American Asthma Foundation.
References
- 1.Strachan DP. Hay fever, hygiene, and household size. Bmj. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cookson JB. Prevalence rates of asthma in developing countries and their comparison with those in Europe and North America. Chest. 1987;91:97s–103s. doi: 10.1378/chest.91.6_supplement.97s. [DOI] [PubMed] [Google Scholar]
- 3.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nature reviews Immunology. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 5.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 6.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clinical and experimental immunology. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. International archives of allergy and immunology. 1992;98:279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- 8.McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends in immunology. 2002;23:450–455. doi: 10.1016/s1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- 9.Kearney JF, Patel P, Stefanov EK, King RG. Natural Antibody Repertoires: Development and Functional Role in Inhibiting Allergic Airway Disease. Annual review of immunology. 2015 doi: 10.1146/annurev-immunol-032713-120140. [DOI] [PubMed] [Google Scholar]
- 10.Stohrer R, Kearney J. Ontogeny of B cell precursors responding to alpha 1- greater than 3 dextran in BALB/c mice. Journal of immunology. 1984;133:2323–2326. [PubMed] [Google Scholar]
- 11.Vakil M, Briles DE, Kearney JF. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Developmental immunology. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton AM, Lehuen A, Kearney JF. Immunofluorescence analysis of B-1 cell ontogeny in the mouse. International immunology. 1994;6:355–361. doi: 10.1093/intimm/6.3.355. [DOI] [PubMed] [Google Scholar]
- 13.Hardy RR. B-1 B cell development. Journal of immunology. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 14.Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. Journal of immunology. 2006;177:6025–6029. doi: 10.4049/jimmunol.177.9.6025. [DOI] [PubMed] [Google Scholar]
- 15.Kin NW, Stefanov EK, Dizon BL, Kearney JF. Antibodies generated against conserved antigens expressed by bacteria and allergen-bearing fungi suppress airway disease. Journal of immunology. 2012;189:2246–2256. doi: 10.4049/jimmunol.1200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel PS, Kearney JF. Neonatal exposure to pneumococcal phosphorylcholine modulates the development of house dust mite allergy during adult life. Journal of immunology. 2015;194:5838–5850. doi: 10.4049/jimmunol.1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in U.S. households. Environmental health perspectives. 2006;114:522–526. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggleston PA, Arruda LK. Ecology and elimination of cockroaches and allergens in the home. The Journal of allergy and clinical immunology. 2001;107:S422–429. doi: 10.1067/mai.2001.113671. [DOI] [PubMed] [Google Scholar]
- 19.Sohn MH, Kim KE. The cockroach and allergic diseases. Allergy, asthma & immunology research. 2012;4:264–269. doi: 10.4168/aair.2012.4.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potera C. Working the bugs out of asthma. Environmental health perspectives. 1997;105:1192–1194. doi: 10.1289/ehp.971051192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomes A, Wunschmann S, Hindley J, Vailes LD, Chapman MD. Cockroach allergens: function, structure and allergenicity. Protein and peptide letters. 2007;14:960–969. doi: 10.2174/092986607782541178. [DOI] [PubMed] [Google Scholar]
- 22.Irani AM. The Relationship Between a Specific IgE Level and Asthma Outcomes: Results From the 2005-2006 National Health and Nutrition Examination Survey. Pediatrics. 2014;134(3):S168. doi: 10.1542/peds.2014-1817HHH. [DOI] [PubMed] [Google Scholar]
- 23.Sarpong SB, Hamilton RG, Eggleston PA, Adkinson NF., Jr Socioeconomic status and race as risk factors for cockroach allergen exposure and sensitization in children with asthma. The Journal of allergy and clinical immunology. 1996;97:1393–1401. doi: 10.1016/s0091-6749(96)70209-9. [DOI] [PubMed] [Google Scholar]
- 24.Gao P. Sensitization to cockroach allergen: immune regulation and genetic determinants. Clinical & developmental immunology. 2012;2012:563760. doi: 10.1155/2012/563760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Jeong KY, Shin KH, Yi MH, Gantulaga D, Hong CS, Yong TS. Reactivity of German cockroach allergen, Bla g 2, peptide fragments to IgE antibodies in patients' sera. The Korean journal of parasitology. 2008;46:243–246. doi: 10.3347/kjp.2008.46.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oseroff C, Sidney J, Tripple V, Grey H, Wood R, Broide DH, Greenbaum J, Kolla R, Peters B, Pomes A, Sette A. Analysis of T cell responses to the major allergens from German cockroach: epitope specificity and relationship to IgE production. Journal of immunology. 2012;189:679–688. doi: 10.4049/jimmunol.1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R, Hershey G, Liu AH, O'Connor GT, Pongracic JA, Zoratti E, Little F, Granada M, Kennedy S, Durham SR, Shamji MH, Busse WW. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. The Journal of allergy and clinical immunology. 2014;133:846–852.e846. doi: 10.1016/j.jaci.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Gustchina A, Glesner J, Wunschmann S, Vailes LD, Chapman MD, Pomes A, Wlodawer A. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. Journal of immunology. 2011;186:333–340. doi: 10.4049/jimmunol.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, Chapman MD, Tomer KB, London RE, Pomes A. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. The Journal of allergy and clinical immunology. 2013;132:1420–1426. doi: 10.1016/j.jaci.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomberg B, Geckeler WR, Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972;177:178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- 31.Beauvais A, Fontaine T, Aimanianda V, Latge JP. Aspergillus cell wall and biofilm. Mycopathologia. 2014;178:371–377. doi: 10.1007/s11046-014-9766-0. [DOI] [PubMed] [Google Scholar]
- 32.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearney JF, McCarthy MT, Stohrer R, Benjamin WH, Jr, Briles DE. Induction of germ-line anti-alpha 1-3 dextran antibody responses in mice by members of the Enterobacteriaceae family. Journal of immunology. 1985;135:3468–3472. [PubMed] [Google Scholar]
- 34.Sutherland IW, Mackenzie CL. Glucan common to the microcyst walls of cyst-forming bacteria. Journal of bacteriology. 1977;129:599–605. doi: 10.1128/jb.129.2.599-605.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries research. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Froscher BG, Klinman NR. Strain-specific silencing of a predominant antidextran clonotype family. J Exp Med. 1985;162:1620–1633. doi: 10.1084/jem.162.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. Journal of immunology. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stohrer R, Kearney JF. Fine idiotype analysis of B cell precursors in the T-dependent and T-independent responses to alpha 1-3 dextran in BALB/c mice. J Exp Med. 1983;158:2081–2094. doi: 10.1084/jem.158.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike IN. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. Journal of immunology. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 40.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 41.Foote JB, Mahmoud TI, Vale AM, Kearney JF. Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. Journal of immunology. 2012;188:57–67. doi: 10.4049/jimmunol.1100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambrecht BN, Hammad H. The immunology of asthma. Nature immunology. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 43.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nature medicine. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nature immunology. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bos NA, Bun JC, Bijma H, Cebra ER, Cebra JJ, Deenen GJ, van der Cammen MJ, Kroese FG. Analysis of IgA-producing hybridomas derived from peritoneal B1 cells. Advances in experimental medicine and biology. 1994;355:265–269. doi: 10.1007/978-1-4615-2492-2_45. [DOI] [PubMed] [Google Scholar]
- 46.Cerutti A. The regulation of IgA class switching. Nature reviews Immunology. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmoud TI, Kearney JF. Terminal deoxynucleotidyl transferase is required for an optimal response to the polysaccharide alpha-1,3 dextran. Journal of immunology. 2010;184:851–858. doi: 10.4049/jimmunol.0902791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabat EA. The upper limit for the size of the human antidextran combining site. Journal of immunology. 1960;84:82–85. [PubMed] [Google Scholar]
- 49.Lundblad A, Steller R, Kabat EA, Hirst JW, Weigert MG, Cohn M. Immunochemical studies on mouse myeloma proteins with specificity for dextran or for levan. Immunochemistry. 1972;9:535–544. doi: 10.1016/0019-2791(72)90063-8. [DOI] [PubMed] [Google Scholar]
- 50.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. The Journal of pathology. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 51.Yel L. Selective IgA deficiency. Journal of clinical immunology. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaffer FM, Monteiro RC, Volanakis JE, Cooper MD. IgA deficiency. Immunodeficiency reviews. 1991;3:15–44. [PubMed] [Google Scholar]
- 53.Schwarze J, Cieslewicz G, Joetham A, Sun LK, Sun WN, Chang TW, Hamelmann E, Gelfand EW. Antigen-specific immunoglobulin-A prevents increased airway responsiveness and lung eosinophilia after airway challenge in sensitized mice. American journal of respiratory and critical care medicine. 1998;158:519–525. doi: 10.1164/ajrccm.158.2.9801014. [DOI] [PubMed] [Google Scholar]
- 54.Smits HH, Gloudemans AK, van Nimwegen M, Willart MA, Soullie T, Muskens F, de Jong EC, Boon L, Pilette C, Johansen FE, Hoogsteden HC, Hammad H, Lambrecht BN. Cholera toxin B suppresses allergic inflammation through induction of secretory IgA. Mucosal immunology. 2009;2:331–339. doi: 10.1038/mi.2009.16. [DOI] [PubMed] [Google Scholar]
- 55.Durham A, Adcock IM, Tliba O. Steroid resistance in severe asthma: current mechanisms and future treatment. Current pharmaceutical design. 2011;17:674–684. doi: 10.2174/138161211795428984. [DOI] [PubMed] [Google Scholar]
- 56.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–1096. doi: 10.1016/S0140-6736(15)00157-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.