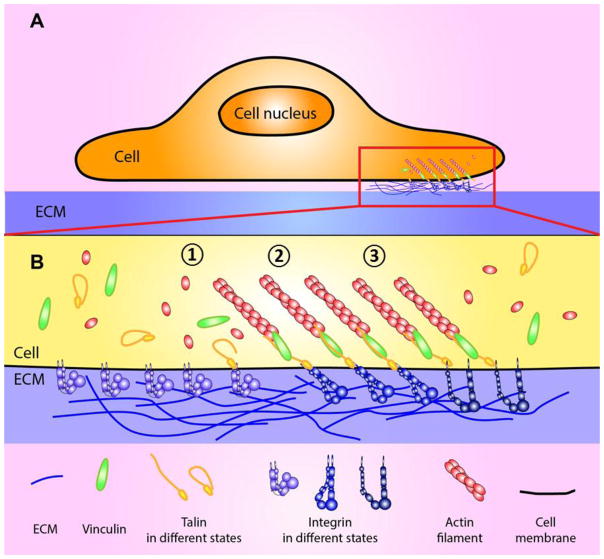
Figure 1. Schematic of the integrated mechanotransduction at the integrin-mediated adhesion site.
(A) Interaction of cell with the ECM, which is mediated by a multi-protein complex, FA. The red rectangle is enlarged in B. (B) A widely accepted model for force-mediated FA maturation with key adaptor molecules. Integrin interacts with its ECM ligands (e.g. fibronectin) and it triggers signal transduction of followings: (1) Integrin in inactivated state. Talin is recruited to the FA site and starts interacting with integrin β3-tail. Vinculin and other adaptor molecules are also recruited and concentration of free G-actin is accumulated at the adhesion site. (2) Integrin engages with its ligand, and transition to intermediate state. F-actin polymerization is accelerated and force is loaded on the linkage of integrin-adaptor molecule to the actin cytoskeleton. Force-activatable molecules (e.g. vinculin and talin) in the adhesion complex sense the externally applied force and modulate their activation and affinity states for their ligands to transduce the mechanical signaling. (3) Conformational activation of integrin, an extended-open high-affinity state, is fully induced by talin-β-integrin tail binding, integrin ligand binding to integrin head piece, or force applied on integrin. The activation results in modulation of interaction kinetics for its ECM ligands to further develop FAs. FA is matured and stabilized at this stage.