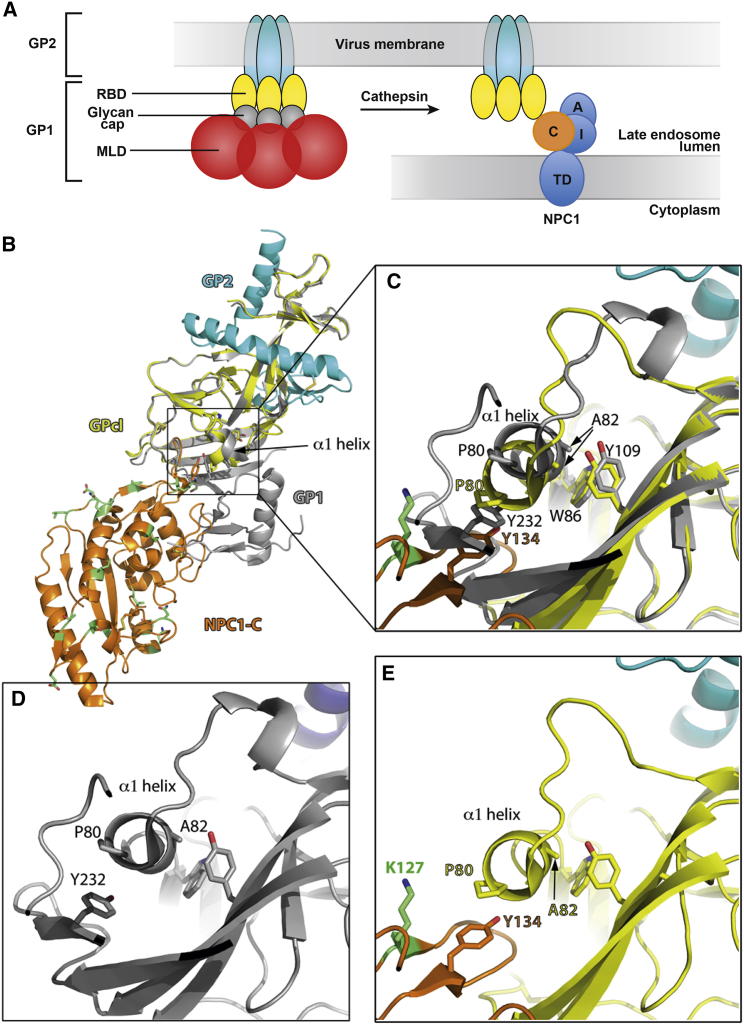
Figure 6.
Residue 82 in the GPcl/NPC1-C Complex
(A) Cartoon showing the organization of the GP complex as anchored in the viral membrane, colored according to domains as labeled (left). The right panel shows the cleaved GP (GPcl), i.e., what remains on the viral membrane after cathepsin cleavage in the endosome, which eliminates the glycan cap and the mucin-like domain (MLD) from the GP trimer. The endosomal membrane and the multiple transmembrane spanning protein NPC1 (Gong et al., 2016) are also shown, with domain C, which is bound by cleaved GP, in orange, contacted by the RBD of GP.
(B) Crystal structure of NPC1-C domain in complex with GPcl (PDB 5F1B [Wang et al., 2016]). NPC1-C is in orange, and residues that are different in bat NPC1-C displayed in green and with sticks. GPcl is shown in yellow, with the GP2 moiety in cyan (as in the cartoon in A). Overlaid is the structure of the GP complex in its pre-fusion form (gray, PDB 3SCY [Lee et al., 2008]), which lacks the MLD but still contains the glycan cap. The side chains of GP1 Y232 and NPC1-C Y134 are shown in sticks. The α1 helix, which moves upon complex formation, is indicated.
(C) Zoom of the region framed in (B), rotated to better display the interactions. The ring of P80, the first residue on the α1 helix, packs against the phenol ring of Y232 within GP1 prior to cathepsin cleavage. Removal of the 191–503 region after cathepsin cleavage frees the α1 helix to interact with NPC1-C, where Y134 takes the same place. The helix packs against the side chains of W86 and Y109, which remain relatively unchanged, while A82 glides downward, accompanying the movement of P80 to maintain the interaction with NPC1-C Y134 in the complex. Because the environment is different in bat NPC1, where residue 127 (labeled in green in (B)) changes from a charged lysine to a hydrophobic isoleucine, our data point to a more favorable interaction with the human NPC1 by acquiring a valine at position 82.
(D and E) Same as (C) but showing, for clarity, only the individual structures in the same orientation.