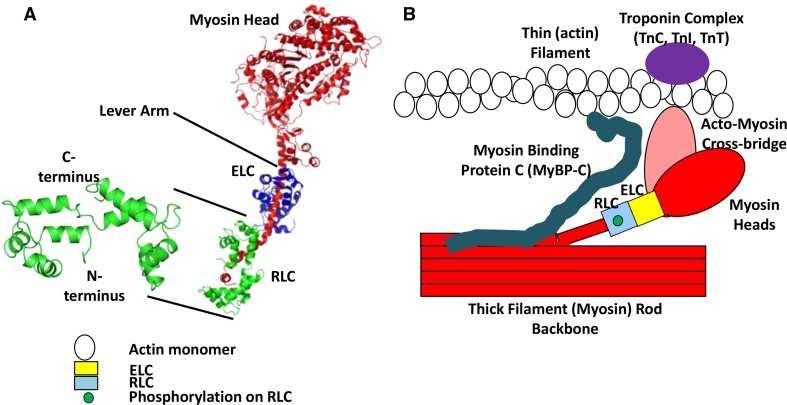
Fig. 1.
RLC structure. a Chicken skeletal muscle isoform of RLC crystal structure with the helix-loop-helix showing four EF hands separated by loops (left). The N- and the C-termini are shown (PDB ID # 2MYS; (Rayment et al. 1993) that wrap around the C-terminus side of the chicken skeletal myosin S1 sub-fragment (right) (Rayment et al. 1993). The N-terminal amino acids 1-20 are missing from the crystal structure due to its flexibility (inherent disorder). The myosin head is the motor domain of the myosin molecule, the site of actin binding and contains the ATP hydrolysis pocket. The regulatory (RLC) and essential (ELC) light chains wrap around the lever arm conferring rigidity and stability to this long alpha-helical rod. b A simplified pictorial representation of the acto-myosin structure with RLC. The diagram shows the thick (myosin containing, red) filament backbone and thin (actin containing, white circles represent actin monomers) filament with an acto-myosin cross-bridge. The ELC (yellow box) and phosphorylated RLC (blue box) are in the neck region of the myosin heavy-chain monomer. The myosin head interacts weakly or strongly with the thin filament forming a cross-bridge structure. The Troponin complex (purple oval) on the thin filament comprising of troponin C, troponin I, and troponin T activate muscle contraction via calcium binding. Myosin binding protein C (MyBP-C) (cyan) is associated with the thick filament backbone and also interacts with the thin filament (not drawn to scale). (Figure is modified from Fig. 6c of Farman et al. 2009)