Fig. 3.
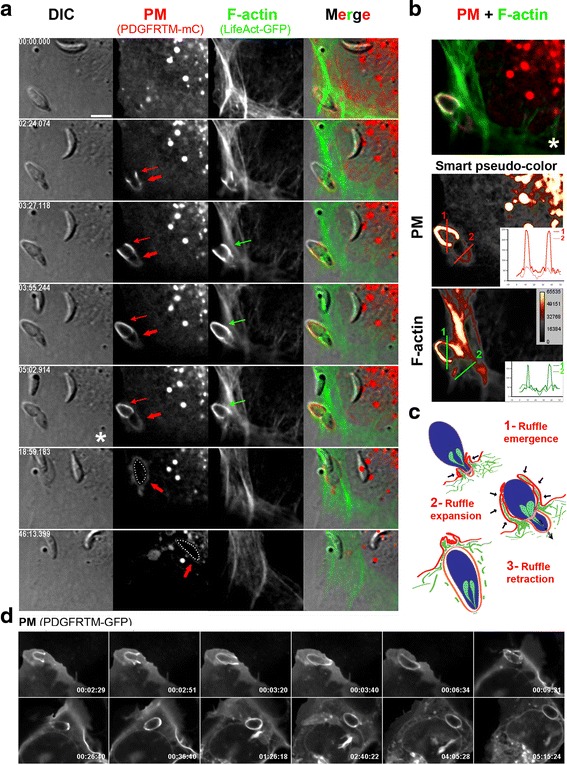
Host cell PM ruffles force ΔMyoA tachyzoites into a primary PV that further traffics in the cytoplasm allowing Toxoplasma development. a, d Time lapses showing DIC and fluorescent sequences of interaction between ΔMyoA tachyzoites and U2OS cells that (b) transiently co-express the fluorescent PM reporter PDGF trans-membrane domain (PDGFTM) coupled to mCherry (mC) and the F-actin binding peptide LifeAct, coupled to GFP (LifeAct-GFP) or (d) transiently express PDGFTM in fusion with GFP. a Note the concomitant development of (i) a PM invagination that remains almost F-actin-negative and that starts at the constriction site (thick red arrows) to eventually surround the internalized parasite and (ii) an outer basket of PM projections (thin red arrows) enriched in F-actin (green arrows). b Overlay between the three layers of the zoite “half in–half out” (top frame) and the pseudocolored image for each fluorescent signal at the same time point (bottom frames). Two transections covering the extracellular (1) and intracellular (2) regions of the parasites allowed us to retrieve signal intensity profiles and show that F-actin is mainly associated with the outer ruffles encircling the extracellular region of the zoite. c Schematic sequence of interaction events leading to progressive enwrapping of the ΔMyoA parasite by host cell actin-driven PM ruffles that push the zoite through the TJ. d Note that the zoite internalized through the wrapping process further develops in the primary vacuole over the 5-h post-entry videorecording. Scale bar: 5 μm
