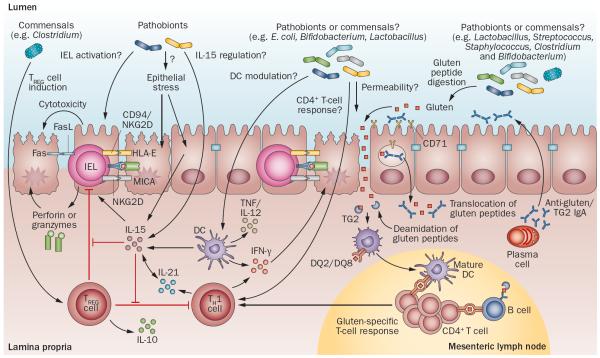
Figure 3.
Potential microbial modulation of coeliac disease pathogenesis. Gluten peptides in the small intestinal lumen translocate the epithelial barrier, via transcellular or paracellular mechanisms and are deamidated by tissue TG2 in the lamina propria. Deamidated gliadin peptides are taken up by lamina propria DCs, inducing a proinflammatory gluten-specific CD4+ T-cell response, characterized by IFN-γ and IL-21 production, and anti-gliadin and anti-TG2 antibody production by B cells in genetically predisposed hosts. Activation of the innate immune response is also a key initial step in coeliac disease. Increased epithelial cell stress can upregulate stress molecules on epithelial cells (HLA-E, MICA/B) and induce IL-15 production from epithelial cells. IL-15 can induce IEL proliferation and activation and cytotoxic killing of epithelial cells, leading to tissue damage. IL-15 can also inhibit the regulatory effects of TREG cells and induce proinflammatory DCs. Microbes, both commensals or opportunistic pathogens (pathobionts), might contribute to the development of coeliac disease by influencing TREG-cell induction, epithelial cell stress, IEL activation or upregulation, IL-15 regulation, DC maturation and proinflammatory cytokine production, intestinal permeability modulation, gluten peptide digestion, and induction of CD4+ T-cell responses. Abbreviations: DC, dendritic cell; FasL, Fas ligand; IEL, intraepithelial lymphocyte; MICA, MHC class I polypeptide-related sequence A; NKG2D, NKG2-D type II integral membrane protein; TCR, T-cell receptor; TG2, tissue transglutaminase 2; TREG cell, regulatory T cell.