Summary
This chapter describes the culture and propagation of murine embryonic stem cells, F9 and P19 and strategies for differentiation of these stem cells into neurons. Protocols focus on maintenance and propagation of these cells and routine procedures employed for differentiation into neuronal cells. Additional protocols are also described for obtaining enriched populations of mature neurons from P19 cells and differentiation of F9 cells into serotonergic or catecholaminergic neurons.
The protocols described herein can be employed for dissection of the pathways such as gliogenesis and neurogenesis that are involved in differentiation of pluripotent stem cells such as F9 and P19 into glial cells or terminally differentiated neurons.
1. Introduction
Embryonal carcinoma (EC) cells are pluripotent stem cells of teratocarcinoma that can differentiate and give rise to all three primary germ layers: endoderm, mesoderm and ectoderm (1). Currently, two mouse teratocarcinoma cell lines, F9 and P19 are widely used for differentiation into neuronal cells (2, 3). Normal spontaneous differentiation of F9 and P19 cells is very low however the differentiation pathway can be induced by addition of retinoic acid (RA) or RA and dibutyryl cyclic AMP (dcAMP) into a variety of cell types including neuronal cells (4–9). There are limitations in the use of RA as a differentiation agent for generation of neuronal cells since studies have shown that P19 cells yield various types of neurons as well as astrocytes, oligodendrocytes, and microglia after treatment with retinoic acid (10). Thus, these stem cells are ideally suited for dissection of the differentiation pathway to a terminally differentiated neural phenotype. However, molecular studies are hindered by the heterogeneity of differentiation.
Therefore, in recent years alternative strategies have been developed for both of these cell lines to induce differentiation into more mature neurons with significant enrichment of neuronal population. It is worth mentioning that neurons derived from the P19 cells treated with RA express functional GABA receptors (11), and ionotropic glutamate receptors of both NMDA and AMPA/kainite types (12). Furthermore, studies demonstrate that neurons derived from P19 cells mature in vivo and capable of displaying neuronal electrophysiological characteristics after four weeks of implantation into rat brains (13, 14). P19 cells have also been used to understand the mechanism of mu-opioid receptor (MOR) upregulation during neuronal differentiation in P19 embryonal carcinoma cells and role of epigenetics MOR up-regulation (15, 16)
This chapter describes the techniques used for maintenance and expansion of both F9 and P19 cells (Basic Protocol A), and routinely used protocol for differentiation into neurons (Basic Protocol B), and then followed by recent protocols that involve modification of basic protocol B to differentiate into more mature enriched neuronal population (Specific protocols).
2. Materials
Prepare all solutions for use in tissue culture using tissue culture grade water. Use of ultrapure water (prepared by purifying deionized water to attain a sensitivity of 18 M Ω cm at 25°C) is highly recommended for all other purposes. Tissue culture wares, flasks and dishes, cryovials. The described procedures are performed in a Class II biological laminar-flow hood. Refrigerated Centrifuge. −20°C and −70°C, Freezers and Tissue culture incubator.
2.1. Cell lines
F9 cells (ATCC, VA, USA, CRL-1720)
P19 cells (ATCC, VA, USA, CRL-1825)
2.2. Reagents
DMEM
α-MEM
Fetal calf serum
New born calf serum
Penicillin/Streptomycin 100X stock solution (10,000 units of penicillin and 10,000 μg of streptomycin per ml)
Neurobasal-A medium
N2 supplement (100X)
Dulbecco’s PBS without calcium and magnesium
All trans-retinoic acid
Ethyl Alcohol
Dibutyryl-cAMP
Cyclohexane carboxylic acid
DMSO
2.3. Growth and differentiation media
F9 growth medium: DMEM, low glucose 90%, fetal calf serum 10%.
P19 growth medium: αMEM 90%, Newborn calf serum 7.5%, fetal calf serum 2.5%.
F9 differentiation medium: DMEM, 95%, fetal calf serum 5%.
P19 differentiation medium: α-MEM, fetal calf serum 5%,
Freeze media: Growth media containing 10% (v/v) tissue culture grade dimethyl sulfoxide (DMSO).
2.4. Preparation of retinoic acid
Dissolve all trans-retinoic acid (RA) in ethanol to a stock solution of 3 mg/ml (0.01 M).
3.5. Preparation of dibutyryl-cAMP (db-cAMP)
db-cAMP is prepared as 10−2 M (10X) or 2 X 10−2 M (20X) stock directly in tissue culture media. Filter the dissolved solution using a 0.2 μm filter before use and is recommended to be used immediately.
3. Methods
3.1 Basic Protocol A
3.1.2 Maintenance and propagation of F9 cells in culture
Remove and discard culture medium and wash cells with Dulbecco-PBS to remove residual serum from culture media.
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Add 8 to 10 ml of complete growth medium to inactivate trypsin and aspirate cells by gently pipetting.
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
Discard supernatant and resuspend cells in 10 ml of F9 growth medium, count number of cells using a Neubauer hemocytometer.
Plate cells at a minimal density of 6 × 105 cells/cm2 in tissue culture dish or tissue culture flask.
Incubate cultures in a humidified tissue culture incubator at 37°C with 5% CO2.
3.1.3. Maintenance and propagation of P19 cells in culture
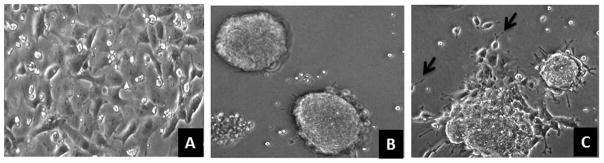
Remove and discard culture medium and wash undifferentiated cells (Fig. 1A) with Dulbecco-PBS to remove residual serum from culture media.
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Add 8 to 10 ml of complete growth medium to inactivate trypsin and aspirate cells by gently pipetting.
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
Discard supernatant and resuspend cells in 10 ml of P19 growth medium, count number of cells using a Neubauer hemocytometer.
Plate cells at a minimal density of 6 × 105 cells/cm2 in tissue culture dish or flask.
Incubate cultures in a humidified tissue culture incubator at 37°C with 5% CO2.
Figure 1. Neural differentiation of P19 murine embryonic carcinoma cells.
A. Undifferentiated P19 cells. B. Embryonic body (EB) formation, P19 cells treated with RA for 2 days and plated on non-adherent Petri dishes. C. EB bodies plated on day 3 on adherent tissue culture dish, note migration of cells from EB and formation of neurites in individual cells (arrows).
3.1.4. Freezing cells
Remove and discard culture medium and wash cells with Dulbecco-PBS to remove residual serum from culture media.
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Add 8 to 10 ml of complete growth medium to inactivate trypsin and aspirate cells by gently pipetting.
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
Discard supernatant and resuspend cells in 5 ml of P19 or F9 growth medium, count number of cells using a Neubauer hemocytometer.
Pellet cells by centrifugation for 5 min at 1000 rpm.
Resuspend cells at 1–2 × 106 cells/ml in freezing medium, mix gently and transfer 1 ml of aliquots into labeled cryovials.
Store cells overnight at −70°C, then transfer vials to liquid nitrogen cryotank for long-term storage.
3.2. Basic Protocol B
3.2.1. Induction of neuronal differentiation of F9 cells
This protocol is widely used to differentiate F9 cells into neurons and is based on a published protocol (17).
Remove culture medium from flask containing F9 cells (75–80% confluency), wash cells with PBS.
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Add 8 to 10 ml of complete growth medium to inactivate trypsin and aspirate cells by gently pipetting.
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
Discard supernatant and resuspend cells in 10 ml of P19 growth medium, count number of cells using a Neubauer hemocytometer.
Plate 1 × 106 cells in 10 ml of F9 differentiation media in tissue culture dish or flask and induce differentiation by adding RA and dcAMP solutions to achieve a final concentration of 10−7 M and 10−3 M, respectively.
Rock the suspension gently to ensure even mixing of cells and RA. Leave the dish/flask undisturbed in a humidified tissue culture incubator at 37°C with 5% CO2 for 10 days.
Change media in the dish/flask every 2–3 days. Continue adding RA and dcAMP after each media change as described in step 6.
3.2.2. Induction of neuronal differentiation of P19 cells
This protocol is used for differentiation of P19 cells into mature neurons and involves generation of cell aggregates popularly known as embryonic bodies (EB) using cells plated on non-adherent culture ware in presence of RA, the aggregates are then plated in presence of RA in regular tissue culture ware.
-
1
Remove culture medium from flask containing P19 cells (75–80% confluency), wash cells with PBS.
-
2
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
-
3
Add 8 to 10 ml of complete growth medium to inactivate trypsin and aspirate cells by gently pipetting.
-
4
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
-
5
Discard supernatant and resuspend cells in 10 ml of P19 growth medium, count number of cells using a Neubauer hemocytometer.
-
6
Plate 1 × 106 cells in 10 ml of P19 differentiation media in 100 × 10mm bacteria grade Petri dish and induce differentiation by adding 1 μl of stock RA solution to 10 ml medium to attain a final concentration of 1 μM RA.
-
8
Rock the suspension gently to ensure even mixing of cells and RA. Leave the petri dishes undisturbed in a humidified tissue culture incubator at 37°C with 5% CO2 for 2 days for formation of EB (Fig.1B).
-
9
On day 2 harvest the EB by transferring them to a 50 ml conical tube and allow the aggregates or EB to settle down at room temperature in the tissue culture hood for 15–20 min.
-
10
Remove the media and then add 10 ml of P19 growth media and transfer cell suspension to regular tissue culture flask or dish and incubate in a humidified tissue culture incubator at 37°C with 5% CO2 for 2 days.
-
11
On day 2 remove the media from culture ware and add 10 ml of P19 differentiation media and incubate in a humidified tissue culture incubator at 37°C with 5% CO2 for 7–8 days, replace half of the media every 2 days to allow maturation of neurons (Fig. 1C).
3.3. Specific protocols
This section describes recent protocols for differentiation of F9 and P19 cells into more mature neurons with significant enrichment of neuronal population.
3.3.1 Protocol for generation of serotonergic or catecholaminergic neurons from F9 cells
This protocol described by Mouillet-Richard et al., (18) utilizes a F9 derived cell line that was generated by introduction of the early genes of SV40 under the control of the adenovirus E1A promoter (19) known as 1C11 clone to differentiate into serotonergic or catecholaminergic neurons. The advantage of using 1C11 cells is that one can attain pure population of neuronal cells that convert within 4 days of induction into serotonergic cells and are able to metabolize, store, and take up serotonin (5-HT)1 (20) and express 5-HT1B/D, 5-HT2A, and 5-HT2B receptors (21). Similarly, with appropriate induction the 1C11 cells within 12 days display a complete catecholaminergic phenotype, coincident with the induction of a functional norepinephrine (NE) uptake.
Remove culture medium from flask containing 1C11 cells (75–80% confluency), wash cells with D-PBS.
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Add 8 to 10 ml of complete F9 growth medium to inactivate trypsin and aspirate cells by gently pipetting.
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
Discard supernatant and resuspend cells in 10 ml of F9 growth medium, count number of cells using a Neubauer hemocytometer.
Plate 4 × 104 cells/cm2 in F9 growth media containing 10% FBS and supplemented with 1 mM dbcAMP and 0.05% cyclohexane carboxylic acid (CCA) in tissue culture dish or flask to differentiate into serotonergic neurons.
Leave the dish/flask in a humidified tissue culture incubator at 37°C with 5% CO2 for 4 days.
Change media in the dish/flask every 2 days. Continue adding 1 mM dbcAMP and 0.05% CCA after each media change as described in step 6.
Plate 4 × 104 cells/cm2 in DMEM supplemented with 5-HT depleted 10% FBS and 1 mM dbcAMP, 0.05% CCA and 2% DMSO in tissue culture dish or flask to differentiate into catecholaminergic neurons.
Leave the dish/flask in a humidified tissue culture incubator at 37°C with 5% CO2 for 12 days.
Change media in the dish/flask every 2 days with DMEM supplemented with 5-HT depleted 10% FBS and 1 mM db-cAMP, 0.05% CCA and 2% DMSO.
3.3.2 Protocol for generation of high-yield enriched neuronal cultures from P19 cells
The cell culture protocol described in the following section is adapted from the protocol described by Monzo et. al. (22) and has been demonstrated to generate a large number of fully mature neurons at both morphological and electrophysiological levels.
Remove culture medium from flask containing P19 cells (75–80% confluency), wash cells with D-PBS.
Add 1–2 ml of 0.25% Trypsin/EDTA solution and observe cells under an inverted microscope until cell layer is dispersed (usually within 5 to 15 minutes).
Add 8 to 10 ml of complete growth medium to inactivate trypsin and aspirate cells by gently pipetting.
Transfer cell suspension into 15 ml conical centrifuge tube and centrifuge cells for 5 min at 1000 rpm.
Discard supernatant and resuspend cells in 10 ml of P19 growth medium, count number of cells using a Neubauer hemocytometer.
Plate 6 × 104 cells/cm2 in P19 differentiation media supplemented with 1 μM RA in tissue culture dish or flask.
Incubate cells in a humidified tissue culture incubator at 37°C with 5% CO2 for 4 days.
Replace media with P19 differentiation media supplemented with 1 μM RA after 2 days.
After 4 days in culture wash the cells 2–3 times with culture medium and trypsinize the cells as described in step 2–4.
Resuspended cell pellet obtained in step 5 in P19 growth medium, re-centrifuge cells and wash cell pellet twice with Neurobasal-A medium without supplements.
Resuspend cells in NBA-medium supplemented with N2 supplement and 2 mM Glutamax.
Plate 9 × 104 cells/cm2 on Matrigel coated plates (see notes for preparing plates, if you wish to coat your own plates.
Incubate plated cells in a humidified tissue culture incubator at 37°C with 5% CO2 for 20 days with medium changes on day 2 and 4 with NBA-medium supplemented with N2 and Glutamax.
On day 5, replace culture medium with NBA-medium supplemented with 2 mM Glutamax, 1×B27, 8 μM Cytosine-d-arabinofuranoside (AraC) and 8 nM 2’-Deoxycytidine (2dCTD; Sigma) for the following 5 days. Replace media every 2 day.
On day 10 replace cell culture medium with NBA-medium supplemented only with 2 mM Glutamax and 1× B27 until day 20. Change cell culture medium every 2 or 3 days.
4. Notes
Culture for both cell lines must be maintained in the exponential growth phase. F9 and P19 cells grow rapidly with a generation time of 10–12 and 12–14 hrs, respectively. Since these cells do not regulate their multiplication in a density-dependent manner it is highly recommended that cells are subcultured at a ratio of 1:10 with renewal of media every 2 to 3 days.
Do not allow cells to be too confluent before the initiation of differentiation.
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal.
Sterile filter high grade ethanol used for dissolving RA. RA is light sensitive to light, wrap tubes containing stock solution with aluminum foil and store it at −80°C for long-term storage. Stock solution is stable for 1 month if stored at −20°C. Vortex the stock solution of RA to ensure that any precipitate that may have formed is re-dissolved. An alternative approach to re-dissolve RA faster is to leave the stock solution at room temperature for 15 min.
An alternative approach of increasing the intracellular concentration of cAMP for differentiation of F9 cells is to use a combination of cholera toxin at a final concentration of 10−10 M and the cyclic phosphodiesterase inhibitor, 3-isobutyl-l-methylxanthine (IBMX) at a final concentration of 10−4 M.
Dilute Matrigel stock solution in the ratio 1:45 in NBA-medium and dispense a volume of 0.1 ml/cm2 of desired plate/flask. Leave plates/flask at room temperature for about 1 h, aspirate the coating solution and leave plates/flask to dry at room temperature for about 15 min. Store coated plates sealed at 4°C.
It is important to deplete 5-HT from medium used for differentiation of P19 cells into serotonergic neurons by dialysis of culture media supplemented with 10% FCS to less than 1 nM.
If desired, the status of neuronal differentiation can be assessed by immunohistochemistry using antibodies for neuronal markers such as β-tubulin III, enolase, neurofilament 200 and MAP2.
Differentiation of 1C11 cells into serotonergic or catecholaminergic neurons can be also assessed by immunohistochemistry using antibodies for 5-HT or norepinephrine, respectively.
Acknowledgments
I am grateful to Dr. Ron Reichel for introducing me to the field of embryonic stem cells and differentiation during the early years of my career. Studies in the author’s laboratory are funded by a grant from NIH/NIDA.
References
- 1.Martin GR. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209:768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- 2.Bernstine EG, Hooper ML, Grandchamp S, Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci USA. 1973;790:3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBurney MW, Rogers BJ. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev Biol. 1982;89:503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- 4.Martin CA, Ziegler LM, Napoli JL. Retinoic acid, dibutyryl-cAMP, and differentiation affect the expression of retinoic acid receptors in F9 cells. Proc Natl Acad Sci USA. 1990;87:4804–4808. doi: 10.1073/pnas.87.12.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253–62. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuff EL, Fewell JW. Induction of neural-like cells and acetylcholinesterase activity in cultures of F9 teratocarcinoma treated with retinoic acid and dibutyryl cyclic adenosine monophosphate. Dev Biol. 1980;77:103–115. doi: 10.1016/0012-1606(80)90459-5. [DOI] [PubMed] [Google Scholar]
- 7.Strickland S, Sawey MJ. Studies on the effect of retinoids on the differentiation of teratocarcinoma stem cells in vitro and in vivo. Dev Biol. 1980;78:76–85. doi: 10.1016/0012-1606(80)90319-x. [DOI] [PubMed] [Google Scholar]
- 8.Liesi P, Rechardt L, Wartiovaara J. Nerve growth factor induces adrenergic neuronal differentiation in F9 teratocarcinoma cells. Nature. 1983;306:265–267. doi: 10.1038/306265a0. [DOI] [PubMed] [Google Scholar]
- 9.Datta PK, Budhiraja S, Reichel RR, Jacob ST. Regulation of ribosomal RNA gene transcription during retinoic acid-induced differentiation of mouse teratocarcinoma cells. Exp Cell Res. 1997;231:198–205. doi: 10.1006/excr.1996.3446. [DOI] [PubMed] [Google Scholar]
- 10.St-Arnaud R, Craig J, McBurney MW, Papkoff J. The int-1 proto-oncogene is transcriptionally activated during neuroectodermal differentiation of P19 mouse embryonal carcinoma cells. Oncogene. 1989;4:1077–1080. [PubMed] [Google Scholar]
- 11.Macpherson PA, Jones S, Pawson PA, Marshall KC, McBurney MW. P19 cells differentiate into Glutamatergic and Glutamate-Responsive Neurons in Vitro. Neuroscience. 1997;80:487–499. doi: 10.1016/s0306-4522(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 12.Magnuson DS, Morassutti DJ, McBurney MW, Marshall KC. Neurons derived from P19 embryonal carcinoma cells develop responses to excitatory and inhibitory neurotransmitters. Brain Res Dev Brain Res. 1995;90:141–150. doi: 10.1016/0165-3806(96)83494-8. [DOI] [PubMed] [Google Scholar]
- 13.Morassutti DJ, Staines WA, Magnuson DS, Marshall KC, McBurney MW. Murine embryonal carcinoma-derived neurons survive and mature following transplantation into adult rat striatum. Neuroscience. 1994;58:753–763. doi: 10.1016/0306-4522(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 14.Magnuson DS, Morassutti DJ, Staines WA, McBurney MW, Marshall KC. In vivo electrophysiological maturation of neurons derived from a multipotent precursor (embryonal carcinoma) cell line. Brain Res Dev Brain Res. 1995;84:130–141. doi: 10.1016/0165-3806(94)00166-w. [DOI] [PubMed] [Google Scholar]
- 15.Hwang CK, Kim CS, Kim do K, Law PY, Wei LN, Loh HH. Up-regulation of the mu-opioid receptor gene is mediated through chromatin remodeling and transcriptional factors in differentiated neuronal cells. Mol Pharmacol. 2010;78:58–68. doi: 10.1124/mol.110.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, Wei LN, Loh HH. Epigenetic programming of mu-opioid receptor gene in mouse brain is regulated by MeCP2 and Brg1 chromatin remodelling factor. J Cell Mol Med. 2009;13:3591–3615. doi: 10.1111/j.1582-4934.2008.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darrow AL, Rickles RJ, Strickland S. Maintenance and use of F9 teratocarcinoma cells. Methods Enzymol. 1990;190:110–117. doi: 10.1016/0076-6879(90)90015-s. [DOI] [PubMed] [Google Scholar]
- 18.Mouillet-Richard S, Mutel V, Loric S, Tournois C, Launay JM, Kellermann O. Regulation by neurotransmitter receptors of serotonergic or catecholaminergic neuronal cell differentiation. J Biol Chem. 2000;275:9186–9192. doi: 10.1074/jbc.275.13.9186. [DOI] [PubMed] [Google Scholar]
- 19.Kellermann O, Kelly F. Immortalization of early embryonic cell derivatives after the transfer of the early region of simian virus 40 into F9 teratocarcinoma cells. Differentiation. 1986;32:74–81. doi: 10.1111/j.1432-0436.1986.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 20.Buc-Caron MH, Launay JM, Lamblin D, Kellermann O. Serotonin uptake, storage, and synthesis in an immortalized committed cell line derived from mouse teratocarcinoma. Proc Natl Acad Sci USA. 1990;87:1922–1926. doi: 10.1073/pnas.87.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellermann O, Loric S, Maroteaux L, Launay JM. Sequential onset of three 5-HT receptors during the 5-hydroxytryptaminergic differentiation of the murine 1C11 cell line. Br J Pharmacol. 1996;118:1161–1170. doi: 10.1111/j.1476-5381.1996.tb15519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monzo HJ, Park TI, Montgomery JM, Faull RL, Dragunow M, Curtis MA. A method for generating high-yield enriched neuronal cultures from P19 embryonal carcinoma cells. J Neurosci Methods. 2012;204:87–103. doi: 10.1016/j.jneumeth.2011.11.008. [DOI] [PubMed] [Google Scholar]