Table 1.
Reaction efficiency correlation with ‘BDFE’.
| |||
|---|---|---|---|
|
| |||
| acid cat | redox cat | ‘BDFE’a | % yieldb |
| none | [Ru(bpy)3](BArF)2 | -- | 0 |
| BzOH | [Ru(bpy)3](BArF)2 | 45 | 0 |
| Et3N•HBF4 | [Ru(bpy)3](BArF)2 | 41 | 0 |
| lutidine•HBF4 | [Ru(bpy)3](BArF)2 | 35 | 0 |
|
| |||
| (PhO)2PO2H | [Ru(bpy)3](BArF)2 | 33 | 78 |
| lutidine•HBF4 | [Ir(ppy)2(dtbbpy)](PF6) | 31 | 74 |
| (PhO)2PO2H | [Ir(ppy)2(dtbbpy)](PF6) | 29 | 93 |
| PTSA | [Ru(bpy)3](BArF)2 | 27 | 92 |
| (PhO)2PO2H | Ir(ppy)3 | 24 | 74 |
Effective ‘BDFE’ values were calculated from the thermodynamic cycle illustrated in Figure 2a using pKa and potential data in MeCN.
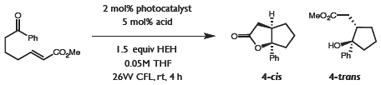
Yields determined by GC analysis of crude reaction mixtures relative to internal standard. Isomeric ratios are ~5:1 in all cases, favoring the cis stereoisomer which spontaneously lactonizes to form product 4-cis.
