Abstract
It is controversial whether tube feeding in people with dementia improves nutritional status or prolongs survival. Guidelines published by several professional societies cite observational studies that have shown no benefit and conclude that tube feeding in patients with advanced dementia should be avoided. However, all studies on tube feeding in dementia have major methodological flaws that invalidate their findings. The present evidence is not sufficient to justify general guidelines. Patients with advanced dementia represent a very heterogeneous group, and evidence demonstrates that some patients with dementia benefit from tube feeding. However, presently available guidelines make a single recommendation against tube feeding for all patients. Clinicians, patients, and surrogates should be aware that the guidelines and prior commentary on this topic tend both to overestimate the strength of evidence for futility and to exaggerate the burdens of tube feeding. Shared decision making requires accurate information tailored to the individual patient's particular situation, not blanket guidelines based on flawed data.
Lay Summary: Many doctors believe that tube feeding does not help people with advanced dementia. Scientific studies suggest that people with dementia who have feeding tubes do not live longer or gain weight compared with those who are carefully hand fed. However, these studies are not very helpful because of flaws in design, which are discussed in this article. Guidelines from professional societies make a blanket recommendation against feeding tubes for anyone with dementia, but an individual approach that takes each person's situation into account seems more appropriate. Patients and surrogates should be aware that the guidelines on this topic tend both to underestimate the benefit and exaggerate the burdens of tube feeding.
Keywords: Dementia, Alzheimer's disease, Feeding tubes, Gastrostomy, Enteral nutrition, Selection bias, Propensity study
Introduction
Tube feeding is considered by the Catholic Church as “in principle, an ordinary and proportionate means of preserving life” (CDF 2007). Research shows that some people with advanced dementia would choose tube feeding if it would prolong their lives or correct malnutrition (Mitchell and Lawson 1999). On the other hand, in Catholic moral teaching a treatment is extraordinary if it is futile. Even treatments that are ordinary in principle can be extraordinary in particular circumstances of futility. The Congregation for the Doctrine of the Faith acknowledges that tube feeding might be futile in some circumstances, including situations when “a patient may be unable to assimilate food and liquids, so that their provision becomes altogether useless” (CDF 2007).
Many claim that tube feeding is futile in patients with advanced dementia. Individual physicians (Cervo, Bryan, and Farber 2006; Finucane, Christmas, and Travis 1999; Gillick 2000), professional societies (AGS Ethics Committee 2014; Barrocas et al. 2010; Fischberg et al. 2013; O'Sullivan Maillet, Baird Schwartz, and Posthauer 2013), and an evidence-based review (Sampson, Candy, and Jones 2009) claim that feeding tubes have not proven beneficial for prolonging survival or correcting malnutrition. For example, the American Geriatric Society (AGS) guidelines on tube feeding in advanced dementia published in 2014 assert that hand feeding “has been shown” by scientific evidence to be as good as tube feeding for outcomes of survival, nutritional status, and others (AGS Ethics Committee 2014). If empirical evidence indeed demonstrates that tube feeding does not improve nutrition or prolong life in patients with advanced dementia, then tube feeding is objectively futile in this population, and thus constitutes morally extraordinary care. But this interpretation of the scientific evidence has not been universally accepted (Regnard et al. 2010), especially in non-Western countries (Jaul, Singer, and Calderon-Margalit 2006; Ribeiro Salomon and Carvalho Garbi Novaes 2015; Shapiro and Friedmann 2006; Shintani 2013).
This discussion will review the evidence on the efficacy of tube feeding in patients with advanced dementia. There is positive evidence that tube feeding is not futile in an appropriately selected patient. A brief critical review of many observational studies will reveal why these studies have been unable to demonstrate a survival benefit for artificial nutrition in dementia. It is not necessarily because tube feeding is futile, but rather because the studies were poorly designed. Current guidelines oversimplify by making a single recommendation against tube feeding for a very heterogeneous group of patients. For some patients with dementia, tube feeding is appropriate, whereas it may be futile for others. Instead of recommending that no patients with dementia receive tube feeding, physicians should review each patient's individual situation to help advise patients and surrogates whether tube feeding might be beneficial. Since it generally cannot be known in advance whether tube feeding will be beneficial in a particular case, patients should usually be advised not to sign an advanced directive to indicate refusal of tube feeding. Instead, an informed healthcare proxy is more appropriate. This literature also provides some guidance as to what prognostic factors might determine which patients are likely to benefit.
What Does It Mean that a Treatment Is “Futile”?
The term “futile” has sometimes been applied loosely in the medical literature to treatments used in dementia patients. For example, some people consider the treatment of pneumonia with antibiotics as “futile,” even though antibiotics are acknowledged to be “fairly routine, not burdensome, relatively inexpensive, and usually effective” and “in the short run ordinary, minimally invasive, relatively painless, and effective” (Sachs, Shega, and Cox-Hayley 2004). This loose idea of futility turns on the fact that, although the treatment is effective to treat the immediate problem, it does not treat the underlying dementia that predisposes a person to infection; and recurrence of infection is common (Brooks et al. 1994; Hedlund et al. 1992; Loeb et al. 1999). The idea here is that the treatment does not change the ultimate cause of the problem or perhaps prolongs an undesirable health state, so the treatment is not really effective in the long run to achieve the patient's goals of care, even though it is effective in the short run. This type of futility can be referred to as subjective futility, since the treatment is considered futile with respect to an individual patient's subjective goals of care. A given treatment could be subjectively futile for one person and subjectively efficacious for another person in the exact same circumstances. (Some may also argue that the burdens associated with sputum culture or other lab testing normally required for proper treatment with antibiotics make the treatment extraordinary. But this is not, strictly speaking, to say that antibiotics are futile, but rather that they are burdensome.)
There is, however, a more strict sense of futility, and this more strict sense will be used here. The goal of a treatment is that which the treatment is ordered to accomplish by its nature. Does the treatment do what it is designed to do? The nature of antibiotic medications directs them to the end of killing bacteria, not curing dementia. The “proper finality” of tube feeding is “providing nourishment to the patient” (Pope St. John Paul II 2004), and tube feeding is considered “useless” when a patient is “unable to assimilate food and liquids” (CDF 2007). Tube feeding is not a cure for underlying dementia or a solution to the underlying swallowing difficulty. We cannot reasonably consider a treatment futile just because it does not work miracles. Tube feeding is a workaround intended to provide nourishment in a person who is otherwise unable to eat adequately. This could be called objective futility, since the treatment is effective or not with respect to an end that is determined by the nature of the treatment and independent of any particular person's subjective goals.
The medical literature on tube feeding in dementia looks at outcomes other than survival and nutritional parameters, e.g., outcomes such as pressure sores and pneumonia. Although they are important, these topics will not be addressed here because they are not, strictly speaking, questions of objective futility. The proper finality of tube feeding is not the prevention of pneumonia, but the provision of nutrition. If tube feeding causes more pneumonia than hand feeding, it is not because tube feeding is relatively futile compared with hand feeding, but rather because it is more burdensome. Pneumonia is a complication of tube feeding, and has nothing to do with its proper end. Similarly, prevention of pressure ulcers is not the proper finality of artificial nutrition. Perhaps one could argue that pressure ulcers represent an indirect measure of nutrition, because malnourished people are more likely to develop them. But these indirect measures are sloppy because they are confounded by many other factors, such as immobility. For our purposes here, there is no need to evaluate an indirect outcome to determine futility when we can easily measure direct parameters, such as weight or albumin.
It may be helpful also to distinguish absolute and relative objective futility. A treatment is absolutely futile if it does not achieve its proper finality at all. A treatment is relatively futile if it achieves its proper finality somewhat, but less effectively than an alternative treatment. This brings us to a second important point. The question of tube feeding only arises when careful hand feeding is not providing adequate nutrition after all other conservative measures (such as nutritional supplements, environmental changes, etc.) are not supplying adequate nutrition. The American Geriatric Society guidelines (AGS Ethics Committee 2014) do not advocate starving a person with dementia. Rather, the guidelines advocate doing the best we can to provide nutrition, namely, through careful hand feeding. The guidelines frame the question as a choice between two means of nutrition. So it is important to note here that the question applies only in those cases where hand feeding has been shown to be inadequate. The scientific literature that will be reviewed here focuses on a question of relative objective futility, which is also the morally relevant sense of futility for tube feeding. Tube feeding is morally extraordinary so long as nutritional intake by mouth is adequate.
Is Tube Feeding Futile in Advanced Dementia?
The AGS guidelines and others (Cervo, Bryan, and Farber 2006; Finucane, Christmas, and Travis 1999; Gillick 2000; Sampson, Candy, and Jones 2009) claim that tube feeding often fails to improve nutritional status. This might be true of aggregate data, although the studies are subject to biases that will be discussed below. But in fact, nutritional parameters demonstrate a wide variety of outcomes in individual patients. For example, one-third to one-half of patients gain weight after feeding tube insertion and an additional 30 to 50 percent maintain stable weight (Arinzon, Peisakh, and Berner 2008; Kaw and Sekas 1994; Peck, Cohen, and Mulvihill 1990). Ciocon et al. found that only about 33 percent of patients continued to lose weight at 6 to 11 months after tube insertion (Ciocon et al. 1988). Arinzon, Peisakh, and Berner (2008) demonstrated improvement in mean albumin, blood counts, creatinine, and other nutritional parameters. Albumin levels increase in a significant percentage (about 20–40%) (Callahan et al. 2000; Kaw and Sekas 1994) and stabilize in up to 88 percent (Ciocon et al. 1988). These studies looked at elderly patients or people in long term care facilities, of which a majority (though not all) had dementia. To say that tube feeding does not improve nutrition in the elderly without qualification ignores the complexity of the data.
Nearly every study of nutritional status reports change in various nutritional parameters from the time of feeding tube insertion compared with some later time. This is an inappropriate outcome measure. Is it success or failure if a patient does not gain weight after feeding tube insertion? Progressive weight loss that stops at the time the feeding tube is inserted indicates the intervention was successful at changing outcome, even if the patient remains stable. He is no longer losing weight. Figure 1 is a hypothetical outcome that clearly illustrates the invalidity of concluding “no change” after percutaneous endoscopic gastrostomy (PEG) means “no benefit” (cf. Löser, Wolters, and Fölsch 1998). The relevant outcome compares slope of weight change before and after tube insertion.
Figure 1.
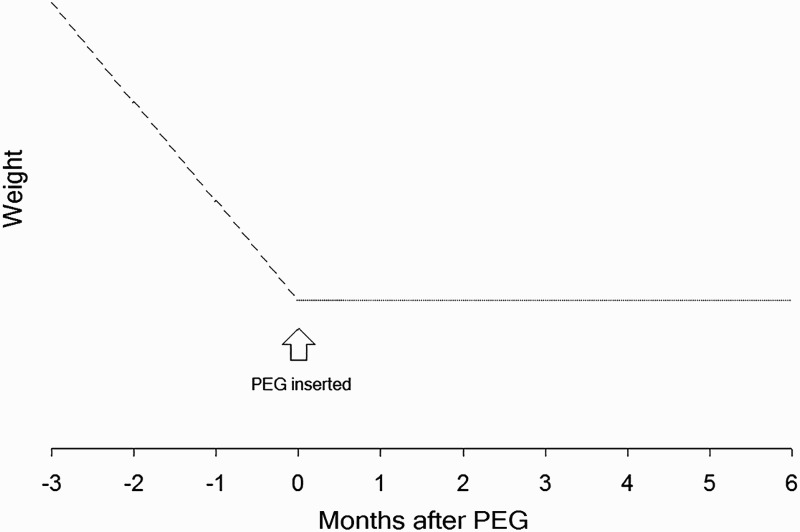
Hypothetical outcome for weight before and after feeding tube insertion. This illustrates that “no change” in a nutritional parameter after PEG does not mean “no benefit.” This type of result, similar to that found by Löser, Wolters, and Fölsch (1998) clearly shows improvement in nutritional status. If data are only collected at baseline and after initiation of enteral nutrition, the benefit will not be apparent. Despite this, almost no studies of nutritional status report data prior to initiation of enteral nutrition.
Thus, empirical studies have shown that some elderly patients in long-term care facilities improve in nutritional status after initiation of tube feeding. The same is true of survival. Tube feeding prolongs survival in some patients, and this will become apparent as we move on to discuss what factors might predict which patients are likely to improve.
What Factors Predict Benefit in Tube Feeding?
The AGS guidelines oversimplify by making a single recommendation for a very heterogeneous group of patients. The guidelines cite high general mortality rates in patients with advanced dementia, but it is important to remember that many patients with dysphagia and dementia are not imminently dying. Predicative models, including Medicare hospice guidelines, have a positive predictive value of only about 30 percent for death within 6 months (Mitchell et al. 2010). About 1 in 4 (Grant, Rudberg, and Brody 1998; Higaki, Yokota, and Ohishi 2008; Shapiro and Friedmann 2006) and perhaps up to 65 percent (Kumagai et al. 2012) of demented patients with gastrostomy live more than 3 years. Many possible risk factors have been found that may separate those for whom tube feeding is beneficial from those for whom it is not. These risk factors can be used to individualize prognosis to facilitate better informed consent for patients with dementia who are considering a feeding tube.
Hospitalization for acute illness at the time of feeding tube decision is a risk factor for poor outcome. Elderly patients who are hospitalized for acute illnesses like pneumonia or stroke have high mortality regardless of whether they have a feeding tube. Abuksis et al. compared PEG outcomes based on referral source. Those referred as an outpatient from the nursing home had a lower 30-day mortality (4%) than those referred with acute illnesses that required hospitalization (29%) despite the fact that the outpatient group had significantly more dementia (87% vs. 46%) (Abuksis et al. 2000). Another study found that median survival of all PEG patients with any diagnosis referred as an inpatient was 161 days, compared with 423 days for dementia patients referred as an outpatient (Rimon, Kagansky, and Levy 2005). One group instituted a policy requiring PEG placement to wait until 30 days after hospital discharge, and 30-day mortality dropped from 51 percent to 15 percent (Abuksis et al. 2004). Cowen, Simpson, and Vettese (1997) developed a model to predict survival based on several risk factors, including age, comorbidity, and baseline serum albumin. Predicted survival at 100 days varied from about 90 percent in the most favorable group to near 0 percent in the least favorable group.
Risk factors for futility of feeding tube in advanced dementia include inpatient status, mild dysphagia, hypoalbuminemia, advanced age, male gender, comorbid illnesses, and perhaps history of pneumonia or gastrectomy (Abuksis et al. 2000; Alvarez-Fernández et al. 2005; Cowen, Simpson, and Vettese 1997; Gaines et al. 2009; Grant, Rudberg, and Brody 1998; Higaki, Yokota, and Ohishi 2008; Martins, Rezende, and Torres 2012; Nair, Hertan, and Pitchumoni 2000; Rimon, Kagansky, and Levy 2005). From this, it is reasonable to think that possible indicators of benefit of tube feeding might include stable outpatient status, younger age, few comorbid illnesses, severe dysphagia, or mild dysphagia with malnutrition despite maximal conservative management. Patients expressing hunger should probably be considered for feeding tube, since this would be a solid indicator that the patient is inclined to bear the burden for the sake of the benefit. A prior trial of nasogastric tube feeding with documented stabilization in nutritional markers would suggest benefit of PEG. Dysphagia due to stroke is a potentially reversible cause of dysphagia.
There is a consensus among ethicists that discussion of benefits and burden of therapy should consider the patient's particular situation (Quill 1989). Indeed, even those who are opposed to tube feeding in advanced dementia in general recognize the need to make exceptions (Brett 2001; Cervo, Bryan, and Farber 2006; Gillick 2000; Grant, Rudberg, and Brody 1998). Current guidelines for tube feeding in advanced dementia do not give enough attention to factors that may help clinicians identify those for whom feeding tubes may in fact prolong life or improve nutritional status.
Why Do Studies Show that Tube Feeding Is Futile?
If tube feeding is beneficial for some patients with advanced dementia, it may seem puzzling that many empirical studies have failed to show benefit of tube feeding. In fact, some think the currently available evidence for futility amounts to a positive proof. Commentary prior to the AGS guidelines issued in 2014 had used cautious absence of evidence language to summarize the data. But now the AGS guidelines claim there is evidence of absence. It “has been shown” that tube feeding is futile.
The hypothesis that tube feeding is futile is a null hypothesis. Typical hypothesis testing assumes a skeptical stance toward the treatment hypothesis in order to avoid false positive errors affirming that a treatment is effective when in fact it is not. This statistical convention makes it intentionally more difficult to prove a treatment effect than to fail to prove it. It also means that failure to reject the treatment hypothesis is not an affirmation of the null. We should view with caution any claim to have proven a null hypothesis, such as the position the AGS guidelines take with the claim that futility “has been shown.”
There are at least 14 observational studies comparing tube feeding with oral feeding in debilitated or elderly patients. Of these, four studies suggested a survival benefit of tube feeding (Cowen, Simpson, and Vettese 1997; Jaul, Singer, and Calderon-Margalit 2006; Rudberg et al. 2000; Shintani 2013), four failed to show benefit (Meier et al. 2001; Mitchell, Kiely, and Lipsitz 1997; Murphy and Lipman 2003; Teno et al. 2012), five suggested harm (Alvarez-Fernández et al. 2005; Arinzon, Peisakh, and Berner 2008; Cintra et al. 2014; Mitchell, Kiely, and Lipsitz 1998; Nair, Hertan, and Pitchumoni 2000), and one study did not report survival data (Peck, Cohen, and Mulvihill 1990). Table 1 summarizes major methodological flaws which invalidate or vitiate the application of their findings to patients with dementia. A series of flawed studies with conflicting results hardly represents “highly consistent empirical work” forming a “preponderance of evidence” (AGS Ethics Committee 2014) against tube feeding.
Table 1.
Methodological flaws in controlled observational studies of tube feeding elderly patients
| Study (year) | Tube survival benefita | Selection bias | Poor or unmatched control group | No group comparison table | Poor inclusion criteria – mild dysphagia | Poor exclusion criteria—too sick (%inpatient) | Cohort not defined by cognitive impairment | Mixed diagnosis in tube group (% dementia) | Equates cognitive impairment and dementia | Mixed tube type | Imprecise measure—database study | Poor outcome measure—tube prior to baseline | Sample size < 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitchell, Kiely, and Lipsitz (1998) | Harm | + | ++ | + | + | + | + (31%) | + | + | + | +++ | ||
| Nair, Hertan, and Pitchumoni (2000) | Harm | + | ++ | + | ? | + (100%) | + | ||||||
| Alvarez-Fernández et al. (2005) | Harm | + | ++ | +/− | +++ | + | + | ||||||
| Arinzon, Peisakh, and Berner (2008) | Harm | + | +++ | ? | ? | …b | + | + | +? | ||||
| Cintra et al. (2014) | Harm | + | +++ | …c | + (84%) | + | + | ||||||
| Mitchell, Kiely, and Lipsitz (1997) | 0 | + | + | + | + | + (53%) | + | + | + | ? | |||
| Meier et al. (2001) | 0 | + | + | …d | ? | + (100%) | + | ||||||
| Murphy and Lipman (2003) | 0 | + | ??? | + | ? | ? | …b | + | |||||
| Teno et al. (2012) | 0 | + | ++ | ++ | + | + | |||||||
| Cowen, Simpson, and Vettese (1997) | Benefit | + | +++ | + | …c | + (100%) | + | + (20%) | |||||
| Rudberg et al. (2000) | Benefit | + | + | …e | + | …f | + | + | + | + | |||
| Jaul, Singer, and Calderon-Margalit (2006) | Benefit | + | +++ | ? | ? | + | + (68%) | ? | + | ||||
| Shintani (2013) | Benefit | + | + | …g | ? | + | + (8–16%) | ?+ | + | ||||
| Peck, Cohen, and Mulvihill (1990) | ? | + | +++ | ? | ? | + | + | + | ++ |
Note: “+” indicates that this flaw affects study design; “?” indicates that insufficient information was reported to determine if this flaw affects study design.
a“Benefit” means tube feeding demonstrated survival benefit. “0” means there was no significant benefit. “Harm“ means tube feeding demonstrated increased mortality. “?” means survival data was not reported.
bLimited to dementia, but vague criteria for diagnosis of dementia.
cExcluded mild dysphagia with formal swallowing evaluation.
dNo direct comparison table, but reports a few selected risk factors for tube placement.
eUsed database variables to select more severe swallowing problems, but no formal swallowing evaluation.
fSelected patients on basis of swallowing problems, but results were similar analyzing subgroup with severe cognitive impairment.
gIncluded mild dysphagia, but reported levels of dysphagia in each group.
As table 1 indicates, there are at least three reasons why observational studies have failed to show an effect of tube feeding. First, the studies are plagued with selection bias. Second, the studies use poor inclusion and exclusion criteria. Third, inappropriate outcome measures were used. Each of these problems would tend to bias the outcome toward the null hypothesis. Let us review each in turn.
Selection Bias
All studies to date are subject to selection bias. There have been no randomized studies on this issue. In every study patients are selected for feeding tube because the patient is thought to be sick enough to need one. Patients who need feeding tubes, then, are sicker as a group than those who do not need them. But a fair comparison of outcomes can only be made when both groups are equally sick. Suppose we want to test whether a sneaker makes people run faster, but we give the sneaker only to the slowest runners, and everyone finishes the race at the same time. It would be wrong to conclude that the sneaker was ineffective, since the intervention was given in a biased way. In fact, if the slower runners finish at the same time as the faster runners, it is evidence that the sneaker was effective. This is the problem of selection bias.
Teno et al. (2012) are sensitive to the problem and attempt to correct for it using complicated statistical methods that include multivariate modeling and propensity weighting. They cross-referenced various databases to select 36,492 nursing home patients with moderate-to-severe dementia who develop need for total assistance with eating, comparing 1,957 patients who received PEG tube feeding with the rest who did not. Essentially, the authors create a model of predicted survival on the basis of many covariates, including the propensity score. The predicted survival curves overlap almost exactly. The authors claim that “Because of the methodological rigor [of their study], healthcare providers can have confidence that feeding tubes do not prolong survival.”
This study deserves in-depth analysis because it is arguably one of the best. It was meant to respond to an editorial (Delegge 2008) questioning the conclusion that tube feeding in dementia does not prolong survival and calling for a randomized trial. Teno et al. (2012) conducted a very large study using a newer statistical method that corrects some of the problems of prior research. Prior studies using the same database had mixed tube types (nasogastric and PEG), but these authors cross referenced with another database to select only PEG tubes. Prior studies had assumed that “cognitive impairment” was synonymous with “dementia,” but this study selected only those patients with a diagnosis of dementia. Some prior studies examined cohorts with prevalent swallowing problems, whereas this study selected only those patients with newly developed (incident) swallowing problems. The authors also use a statistical technique called propensity weighting which is designed to eliminate selection bias. This is why the authors feel their study is rigorous and settles the question. However, a critical evaluation will show that the study, in fact, still exemplifies many of the design flaws that plague research in this area.
Selection bias is operative in the following ways: the tube-fed patients were significantly more likely to be African-American, male, to have diabetes, pneumonia, sepsis, weight loss, swallowing problems, mechanically altered diet, or require nutritional supplements. Other studies have also shown that tube-fed patients are, as a group, more ill than orally fed patients (Alvarez-Fernández et al. 2005; Arinzon, Peisakh, and Berner 2008; Cintra et al. 2014; Kuo et al. 2009; Mitchell, Kiely, and Lipsitz 1997; Nair, Hertan, and Pitchumoni 2000; Peck, Cohen, and Mulvihill 1990).
Teno et al. attempt to correct for selection bias using a propensity score method. Propensity weighting is a relatively new statistical technique designed to eliminate selection bias in observational studies. Speaking very loosely, it is a method of making an observational study more like a randomized trial. Like randomization, it attempts to balance the treated and untreated groups with respect to confounding variables. It is thus important to spend time providing some background on the method in order to evaluate whether Teno et al. have successfully used it.
The propensity score for an individual patient represents his or her probability of receiving treatment based on individual factors. The propensity score becomes a rough estimate for how sick the patient is. Those who are more “sick” are more likely to receive treatment. The propensity score is then used to balance the groups so that they are equally “sick.” There are several different ways of using the propensity score to balance the covariates between the treatment and control samples. Of these, propensity score matching best illustrates how it works to eliminate selection bias. This is not the precise technique used by Teno et al., but it is the easiest to illustrate how a propensity score could possibly eliminate a systematic bias. In a randomized trial, each person has a 50 percent chance of being assigned to treatment. In an observational study, patients are not assigned randomly. We can simulate a random assignment if we take one treated and one untreated patient who each have, say, a 75 percent chance of getting treatment, then another treated and untreated patient who each have a probably of 53 percent, and so on. If every treated patient is matched with an untreated patient with the same probability of receiving treatment, the net effect is similar to randomization, where for every two patients with similar characteristics, one will be treated and one untreated. Figure 2 illustrates this concept graphically. Teno et al. use a propensity technique known as inverse probability of treatment weighting. Austin (2011) provides a helpful introduction to the different types of propensity methods.
Figure 2.
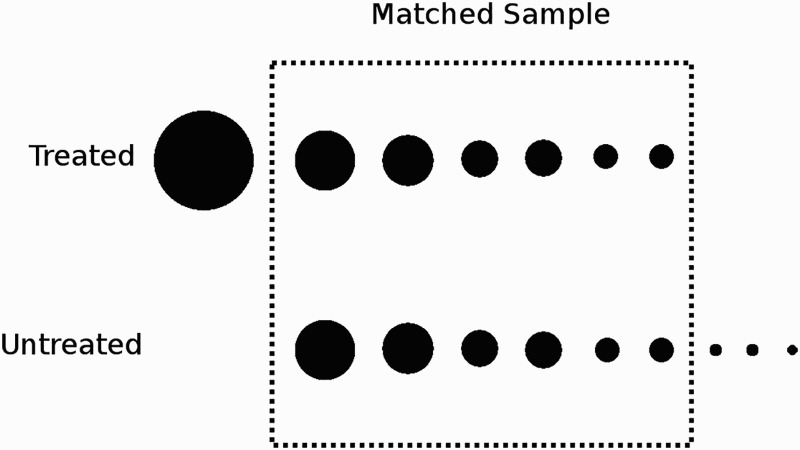
Pictorial illustration of propensity matching. One method of using a propensity score to eliminate selection bias is “propensity matching.” Each circle represents one patient. The size of the circle represents the propensity score (the probability of receiving treatment). Treated patients are matched with an untreated patient with a similar propensity score. The unmatched patients are excluded. Other types of propensity methods incorporate unmatched patients.
Propensity methods, like any statistical technique, rely on the assumption that the statistical model accurately conforms to reality. In this case, the model must accurately estimate the true probability that any given individual will be assigned to receive treatment. For this reason, it is recommended that any propensity study include a diagnostic analysis to show that the treatment and control groups are indeed matched with respect to important covariates (Austin 2009). This is similar to “table 1” of a randomized trial, in which investigators report the balance of covariates to show that the randomization procedure was effective in creating a balanced group. Publishing a propensity study without a diagnostic analysis to prove that the propensity model is correctly specified is like publishing a randomized trial without “table 1.” We have no confidence that selection bias was in fact eliminated. Teno et al. did not report results of a diagnostic analysis. Other propensity studies that do report such an analysis have had residual imbalance in important variables (e.g., Kruse et al. 2004), so it is not a trivial problem. Furthermore, Teno et al.’s methods report the variables entered into the propensity model only in vague, general terms (sociodemographic, etc.), without details about which specific variables were included. Consequently, there is no way to judge whether the model was correctly specified, nor could someone replicate their analysis based on their published methods.
This stands in stark contrast to an excellent propensity study by O'Brien et al. (2015), which publishes the exact variables entered into the model and a diagnostic analysis to show that balance is achieved between treatment and control groups. Interested readers need only compare the two studies to appreciate how poorly Teno et al. report their model. Moreover, Teno et al.’s propensity model almost certainly was specified incorrectly because at least two known variables affecting the outcome of feeding tubes in dementia were not included—inpatient status at the time of feeding tube decision and baseline hypoalbuminemia. As we have seen, inpatient status has a profound effect on outcome so any study that does not consider it in analysis is flawed. Propensity models which do not include important covariates—known or unknown—are not correctly specified (Austin 2009). For these reasons, Teno et al.’s propensity study does not inspire confidence in its negative conclusion. Furthermore, there are other problems aside from the poorly specified and inadequately reported propensity model.
Poor Inclusion–Exclusion Criteria
Teno et al. illustrate another major flaw affecting most studies on this topic. Well-designed trials select patients whom the intervention is most likely to benefit. Proper inclusion and exclusion criteria are of paramount importance in designing trials to determine whether an intervention is successful.
Inclusion of patients with mild dysphagia dilutes the effect size of tube feeding, because they can take more food by mouth. It stands to reason that someone who can take some food by mouth will get less benefit (relatively speaking) from artificial nutrition than someone who can take no food by mouth. The question here is what to do when hand feeding fails, i.e., in cases of severe dysphagia. Any well-designed study of tube feeding should include only those patients with malnutrition despite careful hand feeding. Teno et al. select patients for whom feeding tube is indicated by using a single database variable designed primarily as a measure of cognitive impairment, not dysphagia. This dubious procedure results in inclusion of patients with mild dysphagia in the intervention group. About 1 in 4 patients with PEG in their study did not require a mechanically altered diet at baseline. Only 1 in 4 had weight loss.
In fact, a different group of investigators, Rudberg et al. (2000) found a significant positive survival benefit of tube feeding based on the same database using different selection criteria to restrict the sample to those with more severe dysphagia. This is a retrospective study of survival in 1545 nursing home patients with incident eating problems with and without feeding tubes. Results demonstrated a significant mortality reduction in patients with feeding tubes (39% mortality at 1 year with tube vs. 50% without). The study was primarily a study of dysphagia, not dementia. About 95 percent had cognitive impairment, but it was severe in only about two-thirds. This study methodology is similar to Mitchell, Kiely, and Lipsitz (1997) and other negative studies, except that the results show a benefit to tube feeding. The study is plagued by many of the same methodological problems as other studies, including selection bias, inclusion of hospitalized patients, mixed nasogastric and gastrostomy tube types, some tubes in the “hand fed” group, and equating “cognitive impairment” with dementia. Some of these problems, such as selection bias and inclusion of sick patients, would be likely to bias the results toward the null hypothesis, so these points are less acute when the results are positive. The most serious criticism is that about one-third of the patients did not have severe cognitive impairment. However, a subgroup analysis of only the patients with severe cognitive impairment also demonstrated that “feeding tubes were still associated with significant mortality reduction.” Unfortunately, the details of this sensitivity analysis (including the magnitude of survival benefit) were not reported.
The exclusion of patients with mild dysphagia explains why this study would be positive whereas the others are negative. Like the negative studies, Rudberg et al. (2000) used “total dependence” on others for eating as the primary variable to select patients, but unlike the negative studies, they included patients only if they also had “swallowing problems.” By contrast, only about half of patients in Teno et al.’s (2012) study had “swallowing problems.” The Rudberg group proposed back in 2000 that their study might be positive because they selected patients with more severe dysphagia. It has been over fifteen years, and no one has yet tried to replicate the positive findings using Rudberg's methodology restricted to a dementia cohort. If Teno et al. (2012) want us to “have confidence that feeding tubes do not prolong survival,” they would do well to adapt the methods of the strongest positive study and show that its results cannot be replicated.
Inclusion of mild dysphagia biases toward the null, but so does the opposite problem. Failure to exclude those who are too sick to benefit also reduces the ability to detect a difference. Teno et al., as well as many other studies (Cintra et al. 2014; Cowen, Simpson, and Vettese 1997; Meier et al. 2001; Mitchell, Kiely, and Lipsitz 1997; Mitchell, Kiely, and Lipsitz 1998; Nair, Hertan, and Pitchumoni 2000; Rudberg et al. 2000), include patients who were hospitalized with acute illness at the time the feeding tube was placed. About two-thirds of feeding tubes are inserted during an acute hospitalization (AGS Ethics Committee 2014; Kuo et al. 2009). These patients have high 30-day mortality regardless of whether a feeding tube is used, which would dilute the effect of the feeding tube.
Cintra et al. (2014) used one of the better study designs, but it also has serious problems with selection bias and exclusion criteria. This was a prospective observational study with 6-month follow-up. Patients were eligible only if they carried a diagnosis of possible or probable Alzheimer's disease with advanced disease (FAST stage 7A or worse). Subjects also had to have moderate-to-severe dysphagia, as evaluated by a speech pathologist. Patients with stroke, cancer, or other neurological diseases were excluded. Sample size was predetermined by power calculation and intention-to-treat analysis was used. Results strongly favored the orally fed group for survival. While the inclusion criteria were good, the exclusion criteria were poor. Hospitalized patients were not excluded, and there was a strongly disproportionate number of hospitalized patients in the tube-fed group (84% vs. 39%). A strong selection bias was further evident by the fact that almost one-third of patients in the oral group eventually had a feeding tube placed, implying they selected oral feeding because swallowing problems were initially mild or had not failed a trial of conservative therapy. Indeed, the tube-fed patients had much more advanced dementia. Fifty-four percent of the tube-fed patients were at FAST stage 7E or 7F, compared with 19 percent of the orally fed group (32% vs. 8% for FAST 7F alone). FAST stage 7E means that the patient cannot smile or sit up independently. FAST stage 7F is the most advanced category in the FAST scale, and it indicates the patient cannot hold up his head independently. If 1/3 of the tube-fed patients cannot even hold up their heads (let alone get out of bed), we can understand why the authors found significantly more pressure sores in the tube-fed group, even after statistical correction for some confounding variables. Survival in the tube-fed group was shorter because patients who were closest to death were more often selected for tube feeding. Finally (and almost as an aside compared with the previous points), most patients in this study had nasogastric feeding tubes, which are known to be less effective than gastrostomy for supplying nutrition (Norton et al. 1996; Park et al. 1992).
Many older studies include patients without dementia. As recently as 2014, the AGS guidelines cite Mitchell, Kiely, and Lipsitz (1997) as evidence that tube feeding is futile in advanced dementia, and a widely cited editorial describes this study as “carefully performed” (Gillick 2000). Yet only 53 percent of patients in the tube group actually had dementia, versus 74 percent in the hand-fed group. This fact illustrates problems with both poor inclusion criteria and selection bias. A study in which half of the treated patients do not have the diagnosis of interest is of questionable relevance. Ironically, having dementia was found to be a favorable prognostic sign for survival among those with tube feeding in this study, which is consistent with other research (Abuksis et al. 2000; Gaines et al. 2009; Grant, Rudberg, and Brody 1998; Higaki, Yokota, and Ohishi 2008; Martins, Rezende, and Torres 2012; Mitchell, Kiely, and Lipsitz 1998; Rimon, Kagansky, and Levy 2005).
The importance of good inclusion and exclusion criteria cannot be emphasized strongly enough, since even a randomized controlled trial may yield negative results if inclusion/exclusion criteria are poorly chosen. Statistical correction of selection bias using propensity methods will be all in vain if this fundamental problem is not addressed.
Outcome Measures
Improper or imprecise outcome measures may also lead to false-negative conclusions. It has already been noted how studies of nutritional status often count stabilization of weight as a failure. This will obviously bias results toward the null hypothesis.
Teno et al. illustrate another example of inappropriate outcome measures. The relevant question is whether tube feeding prolongs survival measured from the time the feeding tube is needed. A randomized trial would specify this time point by its inclusion and exclusion criteria. The time of randomization is essentially the point of feeding-tube decision. However, Teno et al. measure survival from onset of dysphagia, not time of feeding-tube decision. In their study, the clock starts ticking as soon as the patient with severe dementia requires total eating assistance. Normally, we would start with a trial of conservative treatments and only consider a feeding tube if the patient continues to be malnourished. Since this “lead time” would be counted for both tube-fed and hand-fed patients, it would tend to reduce the effect size of tube feeding on survival time, and thus bias the results toward the null hypothesis. Even the time of onset of dysphagia is imprecise in their study, because the database they used only measures it every 3 months. Other studies included patients who already had (Alvarez-Fernández et al. 2005; Mitchell, Kiely, and Lipsitz 1998; Peck, Cohen, and Mulvihill 1990) or might have already had (Arinzon, Peisakh, and Berner 2008; Mitchell, Kiely, and Lipsitz 1997) feeding tubes prior to the onset of the study. Clearly, survival time cannot be measured accurately in this situation. Incident, not prevalent, dysphagia is the proper selection criterion.
Summary
Studies to date of feeding tubes in advanced dementia have not, on the whole, confirmed a benefit to tube feeding because of methodological problems in study design, including selection bias, poor patient selection, and poor outcome measures, which tend to bias results toward the null hypothesis. Some of these limitations are due to the nature of the research. Many studies of tube feeding in dementia rely on databases which are, by nature, retrospective and provide limited information.
What Are the Burdens of Tube Feeding?
The focus of this discussion has been on the effectiveness of tube feeding in advanced dementia. However, proving the efficacy of a treatment is only one step in the process of determining whether a treatment is ordinary. Although objective futility entails extraordinariness, efficacy does not necessarily entail ordinariness. Burdens must also be weighed.
A complete discussion of the burdens of tube feeding is beyond the scope of an article focused on futility. However, it would be incomplete to conclude a critical analysis of guidelines on this topic without some brief comments regarding burdens, in order to balance what may be a tendency in this literature to exaggerate the burdens of feeding tube. What follows should be regarded as food for thought rather than a complete discussion of the burdens of tube feeding. It should also be kept in mind that the weighing of burdens should ultimately occur from the patient's perspective. We have to spend time and explore these complex issues with patients and their proxies, and share in the difficult task of advising them as they try to figure out what is best for them.
In reflecting on tube feeding in the vegetative state, the Congregation for the Doctrine of the Faith acknowledges the possibility that artificial nutrition might be excessively burdensome in rare cases (CDF 2007). In the case of a demented patient, though, it might be more commonly extraordinary because a person with dementia retains some ability to take food by mouth, some awareness of his environment, and some ability to pull out the feeding tube. In particular, this raises concerns about the use of restraints to inhibit a patient from pulling out the tube. Physical or chemical restraint is a dehumanizing intervention that looms as a huge potential burden of tube feeding.
There is a possibility that the frequency of restraint has been exaggerated, especially for gastrostomy feeding tubes. The quality of evidence is poor. Research on need for restraint due to feeding tube is complicated by many confounding factors. There are studies that document frequent use of restraints in acutely ill, hospitalized patients with nasogastric feeding tubes (Kvale et al. 2015; Quill 1989). Comparatively, rates are lower with gastrostomy tubes (Ciocon et al. 1988; Lin, Li, and Watson 2011). Nasogastric feeding tubes are irritating to the nose and throat. Gastrostomy tubes by contrast are easier to protect by using an unrestrictive abdominal band. Guidelines are confusing on this subject because they do not differentiate between these two types of tube feeding (AGS Ethics Committee 2014). Restraints are often used in cognitively impaired patients for other reasons, such as to prevent falls and wandering, or to protect a urinary catheter or intravenous line (Mamun and Lim 2005; Raguan, Wolfovitz, and Gil 2015). Reported rates of restraint also depend on whether bedrails are considered restraints. For example, one study reported that 58 percent of patients with advanced dementia were restrained at some point during the last 30 days of life, but only 2 percent had arm restraints whereas 51 percent had bedrails (Di Giulio et al. 2008). Bedrails were obviously not used to prevent removal of a feeding tube. Only 21 percent of these patients were tube fed, and 86 percent of those by nasogastric tube. Cognitively, impaired patients in long-term care facilities and acute care hospitals were often restrained regardless of whether they had a feeding tube (Peck, Cohen, and Mulvihill 1990). Confusion about the reasons for restraint should make us cautious about advising patients that they will be more likely to be restrained if they choose a gastrostomy tube. Rates of restraint use in long term care facilities in the United States were higher prior to introduction of the Omnibus Budget Reconciliation Act (OBRA) of 1987 designed to prevent their use, yet some commentators on this subject rely on older studies (Odom et al. 2003) or studies conducted outside the United States (Teno et al. 2011) when describing the rate of restraint use.
Restraint to protect a gastrostomy tube is probably rare in stable outpatients. Callahan et al. (2000) studied a cohort of ninety-nine patients with PEG due to stroke, dementia, or cancer. Most of them were cognitively impaired. Only about 15 percent were able to complete the mini-mental state exam, and the mean score was 17. Only 2 percent required arm restraints during a 12-month follow-up period. A survey of patients residing at home in Taiwan revealed that 2 of 26 (7.7%) with PEG were restrained, but it does not specify what type of restraint (Lin, Li, and Watson 2011). The authors in that study note that the home care nurses were inexperienced in management of PEG tubes. The rate of restraint in the orally fed cohort was not specified.
The AGS guidelines (2014) claim that “Tube feeding is associated with … greater use of physical and chemical restraints,” citing an old study (Ciocon et al. 1988) as the only reference relevant to restraints. This study does not have an orally fed control group to compare rates of restraint, so the assertion of “greater” use is unfounded. A majority of patients in the study had nasogastric tubes, and Ciocon et al. propose gastrostomy as a means of reducing extubation and thus restraint use. None of sixteen gastrostomy patients were agitated or self-extubated in the long term.
Teno et al. (2011) reported that 25.9 percent of patients in the United States had “hands or upper body tied down to prevent them from pulling at feeding tube” within the last week of life, but these data come from surveys of family members on average two years after the death of the patient. It is doubtful that family members after that period of time would be able to remember and carefully distinguish whether restraints were used for feeding tube, intravenous line, urinary catheter, endotracheal tube, agitation, wandering, or a combination of these factors. It is unclear from the study methods whether the authors limited the study to gastrostomy tubes or included nasogastric tubes and whether they distinguished hospitalized from hospice patients, which are points relevant to the use of restraints as noted above. There was also no control group. The family members of the orally fed patients were not asked about restraints. The recall bias, lack of control group, and lack of other important information about confounders make these data difficult to interpret.
In their discussion section, the authors summarize prior research on restraints and note only two small studies that “reported the use of restraints in persons with a feeding tube, with a study of nursing home residents in Singapore finding that a feeding tube was the reason given for restraint use in one in five nursing home residents” (Teno et al. 2011). The Singapore study (Mamun and Lim 2005) of nursing home patients is of questionable relevance to patients in the United States. In fact, Mamun and Lim explicitly state that their “rate of restraint use was similar to that of American nursing homes before the introduction of OBRA regulations” (emphasis added) and present their research as an argument for changes designed to reduce restraint use. Of ninety-one restrained patients, only twenty-seven had feeding tubes, and only one of these was a gastrostomy tube. The authors suggest placing more PEG as a means of reducing restraint. Mamun and Lim do not specify how many of the demented patients had feeding tubes. But if, for the sake of argument, we make the unlikely but conservative assumption that all patients with feeding tubes also had dementia, it still leaves at least 54 percent of restrained demented patients who had no feeding tube. The high baseline rate of restraint use in this population in the Mamun and Lim study (2005; cf. also Di Giulio et al. 2008) even without feeding tubes necessitates caution before we draw the conclusion that patients will be restrained if they choose a gastrostomy feeding tube and suggest this to them. The unfortunate reality is that, if restraints are needed, they will probably be used regardless of feeding tube status.
The other study cited by Teno et al. (2011) to argue that feeding tubes are associated with increased restraint use is Quill 1989 (also cited by Finucane, Christmas, and Travis 1999; Odom et al. 2003). This is a retrospective review of fifty-five charts of hospitalized patients with stroke, “organic brain syndrome,” or metastatic cancer who received tube feeding. Ninety-three percent had a nasogastric tube at some point, and restraints were used in 53 percent, although the study did not indicate what type of restraints or whether the restraints were used to protect a feeding tube. There was no control group. This uncontrolled, pre-OBRA era study of imminently dying, hospitalized patients with nasogastric tubes provides no accurate indication of whether restraints will be needed for medically stable dementia patients in nursing homes with long-term enteral feeding through gastrostomy.
Peck, Cohen, and Mulvihill (1990) compared fifty-two nursing home patients in 1989 with advanced dementia and tube feeding, with fifty-two orally fed controls. Seventy-five percent had nasogastric tubes. Ninety percent of tube-fed patients had mittens, and 71 percent had some other form of restraint, compared with 56 percent of orally fed patients, but this difference did not reach statistical significance. The orally fed group was much less demented, with 29 percent having a mini-mental state exam (MMSE) score greater than 22, whereas 100 percent of the tube-fed group had MMSE score of 0. These data are mitigated by the high baseline rate of restraint use, grossly unequal disease severity between groups, failure to reach statistical significance, high proportion of nasogastric tubes, and data collection prior to OBRA regulations taking effect.
The literature reviewed above does not seem to warrant the conclusion that long-term feeding via gastrostomy tube commonly leads to use of restraints in demented patients who would otherwise be left unrestrained. Studies primarily pertain to nasogastric feeding, do not have control groups, and do not specify either the type of or reason for restraint. Three small studies of patients with gastrostomy found that restraint was not common over the long term (Callahan et al. 2000; Ciocon et al. 1988; Lin, Li, and Watson 2011), and several authors have recommended increased PEG use as a means to reduce restraints in tube-fed patients (Ciocon et al. 1988; Mamun and Lim 2005).
More research on this topic is needed, and preliminary research should not be difficult. Many recent studies of survival have used the Minimum Data Set database cross-referenced with other governmental databases to identify patients in nursing homes with both cognitive impairment and PEG. These databases include information on the use of restraints. One such study (Kuo et al. 2009) actually reports rates of restraint in tube-fed versus orally fed patients, but unfortunately it seems that only baseline rates prior to receipt of feeding tube were reported. Re-analyzing the raw data from this and similar studies (Teno et al. 2012) should be able to determine whether restraints are more frequently used in gastrostomy patients in nursing homes compared with hand-fed patients. Follow-up studies looking at the type of restraint and the reason for its use may also be necessary. Even if gastrostomy tubes do lead to increased use of restraint, there may be other interventions besides foregoing nutrition that make restraint unnecessary, such as the creative jacket produced by Tamler and Perrin (1992).
Kim (2001) makes an unreferenced claim that “Often, demented patients pull the tubes out.” The author may have been referring to nasogastric tubes, but studies of PEG suggest extubation is more rare than we might think, e.g., 0.8 percent (Callahan et al. 2000), 2.0 percent (Larson et al. 1987), 3.1 percent (Finocchiaro et al. 1997), and 12 percent (Rimon, Kagansky, and Levy 2005). These data for extubation seem consistent with the relatively low rate of arm restraint noted above, although admittedly many patients in these studies did not have dementia. Whether this range of probability constitutes a complication that “often” occurs will be left for the reader to decide.
Other burdens also have been exaggerated. Although complications occur in 50 to 70 percent of patients with tube feeding (Hull et al. 1993; Taylor et al. 1992), 88 percent of these are minor (Taylor et al. 1992). Other studies have found complication rates are about 1.8 complications per patient-year (Barone et al. 2014; Hull et al. 1993). Mechanical complications such as tube blockage can often be resolved as an outpatient (Rimon, Kagansky, and Levy 2005) or at home (Barone et al. 2014). Whereas Kim (2001) claims that “the majority of these tubes require replacement,” actual data from 5209 patients with moderate to severe cognitive impairment reported by Kuo et al. (2009) show that about one in five patients will be sent to the emergency room within the first year of tube replacement for mechanical complications, and these are typically resolved in 2-1/2 hours at the bedside without hospital admission (Odom et al. 2003). While some may consider this too much healthcare utilization, it hardly constitutes a “majority.”
Serious complications are disproportionately reported. A review by Finucane, Christmas, and Travis (1999) is cited by nearly every commentary on this subject. Other reviews parroted and exaggerated Finucane et al. claiming that outlandish, rare complications, such as death or placement of a nasogastric tube into the brain, are complications that “often” occur with tube feeding. For example, “Tube feeding is often associated with complications including infection, bleeding, perforation, fistula, aspiration pneumonia, tube misplacement into other organs (e.g., the lungs or brain), and even death” (Kim 2001). Finucane et al. includes an impressive list of burdens but several of these complications come from case reports (see references 53, 56, 61, 65), which should not be important in the process of informed consent. Reporting of each rare complication (e.g., intussusception, esophageal laceration, gastrocolic fistula, etc.) separately without frequency data and reporting multiple variations on the same theme (e.g., hemothorax, hydrothorax, pneumothorax; pneumonitis, pneumomedianiastinitis, mediastinitis) exaggerates by making serious complications appear more common. Gastrocolic fistula is supported by a secondary reference to Park et al. (1992), which describes it as a “recognized complication” based on Moran, Taylor, and Johnson (1990), who clarify that they were aware of only two cases reported in the literature. Other references are irrelevant. They cite Russell et al. (1996) to defend the claim that both nasogastric and gastrostomy tubes cause reflux. The study is actually a randomized trial of nasogastric tube versus no tube during anesthesia for cardiac surgery. The evidential value of this study is questionable at best for demented patients with gastrostomy not undergoing anesthesia. In terms of informed consent, it may be more appropriate to not overwhelm patients with every possible burden that has been reported in the literature and instead rely on outcomes from large series.
There are double standards. Gastrointestinal symptoms such as nausea, vomiting, diarrhea, weight loss, anorexia, or electrolyte disturbances are called “side effects” when caused by pain medications used in palliative care, but are counted as “complications” of tube feeding (Barone et al. 2014; Finucane, Christmas, and Travis 1999; Gutierrez and Balfe 1991; Hull et al. 1993; Moran, Taylor, and Johnson 1990). These often resolve by changing feed formula without need for hospitalization (Hull et al. 1993). The reduction in time needed for feeding is cited as a burden of tube feeding in dementia (AGS Ethics Committee 2014; Finucane, Christmas, and Travis 1999), whereas it is often perceived as a benefit for patients with amyotrophic lateral sclerosis (Mitsumoto and Rabkin 2007). Social interaction associated with mealtimes and pleasure of taking food by mouth persist, despite tube feeding, in about 50 percent of patients (Callahan et al. 2000); and this percentage could probably be increased with education and a little effort. In some settings, especially patients cared for at home, gastrostomy feeding may promote enjoyable eating by reducing worry about adequate oral intake (Regnard et al. 2010).
This brief excursion into burdens is not intended to be an exhaustive discussion. More can be said, for example, about the frequency of pneumonia or pressure ulcers in artificially fed patients with dementia, which would each require a separate review of its own. The main point here is to comment on the guidelines and address a few specific areas that may suggest a tendency to emphasize burdens in the literature on this topic.
Conclusion
There is reason to believe that some patients with advanced dementia can benefit from tube feeding. Physicians are obligated to consider each patient's individual medical situation and perspective on burdens when making a recommendation for or against a specific medical therapy. It is inappropriate for guidelines to issue a blanket statement against the use of feeding tubes when in fact feeding tubes help some people. Individual physicians and professional societies have not approached observational studies of tube feeding with a sufficiently critical mindset, and current guidelines overstate the strength of the scientific evidence. While there are many observational studies that suggest feeding tubes do not improve length of life, these studies are subject to design flaws that probably invalidate the findings. We are still in the hypothesis formation stage with respect to this question, rather than the guideline formation stage. Additionally, the guidelines overemphasize potential burdens, especially the use of restraints.
It is important to emphasize that these points are fully compatible with the patient's (or surrogate's) right and responsibility to determine for him- or herself whether tube feeding constitutes proportionate care in light of his or her specific situation. The considerations addressed here do not definitively answer the scientific question of how often (or rarely) tube feeding is beneficial in advanced dementia. Even for treatments that are considered ordinary in principle, there remains a subjective element to the determination of proportionality. It is the patient's perspective that determines whether a given burden is proportionate to a given benefit. However, the subjective element has limits and does not mean that a decision is right just because a patient makes it. With rights come responsibilities. The patient has a responsibility to inform his conscience by learning the relevant moral principles, to apply them to his own case after carefully considering the advice and expertise of the doctor, and to judge accurately his own moral strength. But in the end it is the patient's decision, and it is not the physician's role to impose a decision paternalistically.
The patient bears the lion's share of responsibility for determining whether a treatment is proportionate and mandatory, but the doctor sometimes plays more than an advisory role in determining a treatment to be optional. The Declaration on Euthanasia asserts that a doctor may “judge that the investment in instruments and personnel is disproportionate to the results foreseen” (CDF 1980). A doctor is a free person too, and cannot be forced in conscience to offer care he or she deems disproportionate, including futile treatment. If tube feeding in advanced dementia is falsely thought to be futile, physicians may not offer this beneficial treatment. There is evidence that this in fact occurs. Citing the studies critiqued here as their evidence, Mitchell et al. (2012) claim that tube feeding is a marker for poor quality end-of-life care in dementia. They chart a way forward that includes a systematic effort to reduce tube feeding in populations more likely to choose it, such as African-Americans and the southeast United States, even though the authors admit that these choices are based partly on “individual preferences” and “cultural influences.” These authors judge tube feeding to be objectively disproportionate care, and thus they argue we should take steps to limit its use even when a patient's preferences would be inclined to accept it. This would be ethically justified only if tube feeding were indeed futile across the board. But as I have attempted to show, there is good reason to doubt that.
Now someone could argue that tube feeding is no different than any other medical treatment, and thus needs to be justified by positive evidence of benefit before it can be recommended. But this objection fails for three reasons. First, it has been noted above that there are subpopulations for which the evidence suggests that it is beneficial. This includes a positive study in nursing home patients with severe dysphagia (Rudberg et al. 2000). Second, it is a double standard. There has been no systematic attempt to determine for whom tube feeding might be beneficial. In most cases of a negative trial, experts comb the data to see if perhaps the treatment might work in a subpopulation. But this aggressive approach has not occurred with tube feeding. Teno et al. (2012) did analyze subgroups of patients who received tube feedings early or late after developing total eating dependence. But they did not analyze other, more interesting subgroups, such those with weight loss, mechanically altered diet, or “swallowing problems.” (The latter would have replicated the methodology in Rudberg et al. [2000].) Instead, many thought leaders in dementia seem content to let negative trials stand. For example, the above mentioned paper by Mitchell et al. (2012) proposes that we research means to encourage tube refusal rather than try to find out for whom artificial nutrition is beneficial. Historically, some commentators have been quick to condemn tube feeding on the basis of scant evidence. Finucane, Christmas, and Travis (1999) denounced tube feeding in advanced dementia at a time when few studies had been done. If we are going to treat artificial nutrition as we do other potentially beneficial treatments, we should be consistent and review the data to determine why the trial was negative with the goal of ultimately conducting a positive trial. Third, tube feeding is not like other medical treatments. The act of feeding a person is, in principle, ordinary care. Now “in principle” ordinary does not mean “always” ordinary. But it does mean that the burden of proof is on those who say that it is futile. Since there is a presumption in favor of providing care that is, in principle, ordinary, opponents of that care must demonstrate that it does not work. This is unlike other medical treatments which do not fall into the class of acts that are ordinary in principle.
I would argue, instead, that higher quality research is needed. Ten flawed studies do not equal a good study. Repeating studies with the same essential methodology and same flaws does not prove that tube feeding is futile. The studies discussed here raise some interesting questions about the benefit of tube feeding, but should not be cited to patients as definitive proof of the hypothesis that tube feeding does not prolong survival. This kind of research is preliminary and provocative. The level of evidence might be sufficient to create equipoise justifying a randomized controlled trial. But it is not sufficient to justify general guidelines (AGS Ethics Committee 2014) and policy changes (Mitchell et al. 2012; Monteleoni and Clark 2004). Future research should include well-done propensity studies, with an aim to determine whether there is a subpopulation for whom tube feeding is most appropriate. Observational studies with better design, accounting for the problems outlined here, are required to form sufficient equipoise to justify a randomized trial. For example, a prospective observational study of Alzheimer's patients with severe dysphagia, documented by a speech therapist, who were not recently hospitalized may reveal patients for whom there is a clear survival and nutritional advantage without resorting to a randomized trial. We should not prematurely take tube feeding off the table as an option. A comprehensive presentation of its benefits and potential burdens should be presented to patients (or their surrogates) for their own determination.
Biography
Matthew C. Lynch, M.D., studied medicine at the University of Rochester, NY, and then completed a neurology residency at the University of Cincinnati, OH, and a clinical neurophysiology fellowship at Dartmouth-Hitchcock Medical Center. He now practices neurology with Nathan Littauer Hospital in Gloversville, NY.
References
- Abuksis G., Mor M., Plaut S., Fraser G., and Niv Y.. 2004. Outcome of percutaneous endoscopic gastrostomy (PEG): Comparison of two policies in a 4-year experience. Clinical Nutrition 23: 341–6. doi: 10.1016/j.clnu.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Abuksis G., Mor M., Segal N., Shemesh I., Plout S., Sulkes J., Fraser G. M., and Niv Y.. 2000. Percutaneous endoscopic gastrostomy: High mortality rates in hospitalized patients. American Journal of Gastroenterology 95: 128–32. doi: 10.1111/j.1572-0241.2000.01672.x [DOI] [PubMed] [Google Scholar]
- Alvarez-Fernández B., García-Ordoñez M. A., Martínez-Manzanares C., and Gómez-Huelgas R.. 2005. Survival of a cohort of elderly patients with advanced dementia: Nasogastric tube feeding as a risk factor for mortality. International Journal of Geriatric Psychiatry 20: 363–70. doi: 10.1002/gps.1299 [DOI] [PubMed] [Google Scholar]
- American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee 2014. American geriatrics society feeding tubes in advanced dementia position statement. Journal of the American Geriatrics Society 62: 1590–1593. doi: 10.1111/jgs.12924 [DOI] [PubMed] [Google Scholar]
- Arinzon Z., Peisakh A., and Berner Y. N.. 2008. Evaluation of the benefits of enteral nutrition in long-term care elderly patients. Journal of the American Medical Directors Association 9: 657–62. doi: 10.1016/j.jamda.2008.06.002 [DOI] [PubMed] [Google Scholar]
- ASPEN Ethics Position Paper Task Force, Barrocas A., Geppert C., Durfee S. M., Maillet J. O., Monturo C., Mueller C., Stratton K., Valentine C., and ASPEN Board of Directors; American Society for Parenteral and Enteral Nutrition . 2010. A.S.P.E.N. ethics position paper. Nutrition in Clinical Practice 25: 672–9. doi: 10.1177/0884533610385429 [DOI] [PubMed] [Google Scholar]
- Austin P. C. 2009. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Medical Decision Making 29: 661–77. doi: 10.1177/0272989X09341755 [DOI] [PubMed] [Google Scholar]
- Austin P. C. 2011. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research 46: 399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone M., Viggiani M. T., Amoruso A., Licinio R., Iannone A., Montenegro L., Scavo M. P., Addante I., and Di Leo A.. 2014. Influence of age and type of underlying disease on complications related to home enteral nutrition: A single Italian center experience. Journal of Parenteral and Enteral Nutrition 38: 991–5. doi: 10.1177/0148607113498422 [DOI] [PubMed] [Google Scholar]
- Brett A. S. 2001. Dementia, gastrostomy tubes, and mortality. Archives of Internal Medicine 161: 2385–6. doi: 10.1001/archinte.161.19.2385-a [DOI] [PubMed] [Google Scholar]
- Brooks S., Warshaw G., Hasse L., and Kues J. R.. 1994. The physician decision-making process in transferring nursing home patients to the hospital. Archives of Internal Medicine 154: 902–8. doi: 10.1001/archinte.1994.00420080110011 [DOI] [PubMed] [Google Scholar]
- Callahan C. M., Haag K. M., Weinberger M., Tierney W. M., Buchanan N. N., Stump T. E., and Nisi R.. 2000. Outcomes of percutaneous endoscopic gastrostomy among older adults in a community setting. Journal of the American Geriatrics Society 48: 1048–54. doi: 10.1111/j.1532-5415.2000.tb04779.x [DOI] [PubMed] [Google Scholar]
- Cervo F. A., Bryan L., and Farber S.. 2006. To PEG or not to PEG: A review of evidence for placing feeding tubes in advanced dementia and the decision-making process. Geriatrics 61: 30–35. [PubMed] [Google Scholar]
- Cintra M. T., de Rezende N. A., de Moraes E. N., Cunha L. C., and da Gama Torres H. O.. 2014. A comparison of survival, pneumonia, and hospitalization in patients with advanced dementia and dysphagia receiving either oral or enteral nutrition. Journal of Nutrition, Health & Aging 18: 894–9. doi: 10.1007/s12603-014-0487-3 [DOI] [PubMed] [Google Scholar]
- Ciocon J. O., Silverstone F. A., Graver L. M., and Foley C. J.. 1988. Tube feedings in elderly patients. Indications, benefits, and complications. Archives of Internal Medicine 148: 429–33. doi: 10.1001/archinte.1988.00380020173022 [DOI] [PubMed] [Google Scholar]
- Congregation for the Doctrine of the Faith (CDF) 1980. Declaration on Euthanasia.
- Congregation for the Doctrine of the Faith (CDF) 2007. Commentary on Responses to Certain Questions of the United States Conference of Catholic Bishops concerning Artificial Nutrition and Hydration.
- Cowen M. E., Simpson S. L., and Vettese T. E.. 1997. Survival estimates for patients with abnormal swallowing studies. Journal of General Internal Medicine 12: 88–94. doi: 10.1007/s11606-006-5002-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delegge M. H. 2008. Percutaneous endoscopic gastrostomy in the dementia patient: Helpful or hindering? American Journal of Gastroenterology 103: 1018–20. doi: 10.1111/j.1572-0241.2007.01701.x [DOI] [PubMed] [Google Scholar]
- Di Giulio P., Toscani F., Villani D., Brunelli C., Gentile S., and Spadin P.. 2008. Dying with advanced dementia in long-term care geriatric institutions: A retrospective study. Journal of Palliative Medicine 11: 1023–28. doi: 10.1089/jpm.2008.0020 [DOI] [PubMed] [Google Scholar]
- Finocchiaro C., Galletti R., Rovera G., Ferrari A., Todros L., Vuolo A., and Balzola F.. 1997. Percutaneous endoscopic gastrostomy: A long-term follow-up. Nutrition 13: 520–523. doi: 10.1016/S0899-9007(97)00030-0 [DOI] [PubMed] [Google Scholar]
- Finucane T. E., Christmas C., and Travis K.. 1999. Tube feeding in patients with advanced dementia: A review of the evidence. JAMA 282: 1365–70. doi: 10.1001/jama.282.14.1365 [DOI] [PubMed] [Google Scholar]
- Fischberg D., Bull J., Casarett D., Hanson L. C., Klein S. M., Rotella J., Smith T., Storey C. P. Jr., Teno J. M., Widera E., and HPM Choosing Wisely Task Force . 2013. Five things physicians and patients should question in hospice and palliative medicine. Journal of Pain and Symptom Management 45: 595–605. doi: 10.1016/j.jpainsymman.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Gaines D. I., Durkalski V., Patel A., and DeLegge M. H.. 2009. Dementia and cognitive impairment are not associated with earlier mortality after percutaneous endoscopic gastrostomy. Journal of Parenteral and Enteral Nutrition 33: 62–6. doi: 10.1177/0148607108321709 [DOI] [PubMed] [Google Scholar]
- Gillick M. R. 2000. Rethinking the role of tube feeding in patients with advanced dementia. New England Journal of Medicine 342: 206–10. doi: 10.1056/NEJM200001203420312 [DOI] [PubMed] [Google Scholar]
- Grant M. D., Rudberg M. A., and Brody J. A.. 1998. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA 279: 1973–6. doi: 10.1001/jama.279.24.1973 [DOI] [PubMed] [Google Scholar]
- Gutierrez E. D., and Balfe D. M.. 1991. Fluoroscopically guided nasoenteric feeding tube placement: Results of a 1-year study. Radiology 178: 759–62. doi: 10.1148/radiology.178.3.1899727 [DOI] [PubMed] [Google Scholar]
- Hedlund J. U., Ortqvist A. B., Kalin M., Scalia-Tomba G., and Giesecke J.. 1992. Risk of pneumonia in patients previously treated in hospital for pneumonia. Lancet 340: 396–7. doi: 10.1016/0140-6736(92)91473-L [DOI] [PubMed] [Google Scholar]
- Higaki F., Yokota O., and Ohishi M.. 2008. Factors predictive of survival after percutaneous endoscopic gastrostomy in the elderly: Is dementia really a risk factor? American Journal of Gastroenterology 103: 1011–6. quiz 1017. doi: 10.1111/j.1572-0241.2007.01719.x [DOI] [PubMed] [Google Scholar]
- Hull M. A., Rawlings J., Murray F. E., Field J., McIntyre A. S., Mahida Y. R., Hawkey C. J., and Allison S. P.. 1993. Audit of outcome of long-term enteral nutrition by percutaneous endoscopic gastrostomy. Lancet 341: 869–72. doi: 10.1016/0140-6736(93)93072-9 [DOI] [PubMed] [Google Scholar]
- Jaul E., Singer P., Calderon-Margalit R.. 2006. Tube feeding in the demented elderly with severe disabilities. Israel Medical Association Journal 8: 870–874. [PubMed] [Google Scholar]
- John Paul Pope., II 2004. Address to the Congress on Life-Sustaining Treatments and Vegetative State. March 20.
- Kaw M., and Sekas G.. 1994. Long-term follow-up of consequences of percutaneous endoscopic gastrostomy (PEG) tubes in nursing home patients. Digestive Diseases and Sciences 39: 738–43. doi: 10.1007/BF02087416 [DOI] [PubMed] [Google Scholar]
- Kim Y. I. 2001. To feed or not to feed: Tube feeding in patients with advanced dementia. Nutrition Reviews 59: 86–8. doi: 10.1111/j.1753-4887.2001.tb06994.x [DOI] [PubMed] [Google Scholar]
- Kruse R. L., Mehr D. R., Boles K. E., Lave J. R., Binder E. F., Madsen R., and D'Agostino R. B.. 2004. Does hospitalization impact survival after lower respiratory infection in nursing home residents? Medical Care 42: 860–870. doi: 10.1097/01.mlr.0000135828.95415.b1 [DOI] [PubMed] [Google Scholar]
- Kumagai R., Kubokura M., Sano A., Shinomiya M., Ohta S., Ishibiki Y., Narumi K., Aiba M., and Ichimiya Y.. 2012. Clinical evaluation of percutaneous endoscopic gastrostomy tube feeding in Japanese patients with dementia. Psychiatry and Clinical Neurosciences 66: 418–22. doi: 10.1111/j.1440-1819.2012.02378.x [DOI] [PubMed] [Google Scholar]
- Kuo S., Rhodes R. L., Mitchell S. L., Mor V., and Teno J. M.. 2009. Natural history of feeding-tube use in nursing home residents with advanced dementia. Journal of the American Medical Directors Association 10: 264–70. doi: 10.1016/j.jamda.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvale E., Dionne-Odom J. N., Redden D. T., Bailey F. A., Bakitas M., Goode P. S., Williams B. R., Haddock K. S., and Burgio K. L.. 2015. Predictors of physical restraint use in hospitalized veterans at end of life: An analysis of data from the BEACON trial. Journal of Palliatiative Medicine 18: 520–526. doi: 10.1089/jpm.2014.0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. E., Burton D. D., Schroeder K. W., and DiMagno E. P.. 1987. Percutaneous endoscopic gastrostomy. Indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology 93: 48–52. doi: 10.1016/0016-5085(87)90312-X [DOI] [PubMed] [Google Scholar]
- Lin L. C., Li M. H., and Watson R.. 2011. A survey of the reasons patients do not chose percutaneous endoscopic gastrostomy/jejunostomy (PEG/PEJ) as a route for long-term feeding. Journal of Clinical Nursing 20: 802–10. doi: 10.1111/j.1365-2702.2010.03541.x [DOI] [PubMed] [Google Scholar]
- Loeb M., McGeer A., McArthur M., Walter S., and Simor A. E.. 1999. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Archives of Internal Medicine 159: 2058–64. doi: 10.1001/archinte.159.17.2058 [DOI] [PubMed] [Google Scholar]
- Löser C., Wolters S., and Fölsch U. R.. 1998. Enteral long-term nutrition via percutaneous endoscopic gastrostomy (PEG) in 210 patients: A four-year prospective study. Digestive Diseases and Sciences 43: 2549–57. doi: 10.1023/A:1026615106348 [DOI] [PubMed] [Google Scholar]
- Mamun K., and Lim J.. 2005. Use of physical restraints in nursing homes: Current practice in Singapore. Annals of the Academy of Medicine, Singapore 34: 158–62. [PubMed] [Google Scholar]
- Martins A. S., Rezende N. A., and Torres H. O.. 2012. Occurrence of complications and survival rates in elderly with neurological disorders undergoing enteral nutrition therapy. Revista da Associação Médica Brasileira 58: 691–7. doi: 10.1016/S0104-4230(12)70273-7 [DOI] [PubMed] [Google Scholar]
- Meier D. E., Ahronheim J. C., Morris J., Baskin-Lyons S., and Morrison R. S.. 2001. High short-term mortality in hospitalized patients with advanced dementia: Lack of benefit of tube feeding. Archives of Internal Medicine 161: 594–9. doi: 10.1001/archinte.161.4.594 [DOI] [PubMed] [Google Scholar]
- Mitchell S. L., Black B. S., Ersek M., Hanson L. C., Miller S. C., Sachs G. A., Teno J. M., and Morrison R. S.. 2012. Advanced dementia: State of the art and priorities for the next decade. Annals of Internal Medicine 156: 45–51. doi: 10.7326/0003-4819-156-1-201201030-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. L., Kiely D. K., and Lipsitz L. A.. 1997. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Archives of Internal Medicine 157: 327–32. doi: 10.1001/archinte.1997.00440240091014 [DOI] [PubMed] [Google Scholar]
- Mitchell S. L., Kiely D. K., and Lipsitz L. A.. 1998. Does artificial enteral nutrition prolong the survival of institutionalized elders with chewing and swallowing problems? Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 53: 207–13. doi: 10.1093/gerona/53A.3.M207 [DOI] [PubMed] [Google Scholar]
- Mitchell S. L., and Lawson F. M.. 1999. Decision-making for long-term tube-feeding in cognitively impaired elderly people. CMAJ 160: 1705–9. [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. L., Miller S. C., Teno J. M., Kiely D. K., Davis R. B., and Shaffer M. L.. 2010. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA 304: 1929–35. doi: 10.1001/jama.2010.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto H., and Rabkin J. G.. 2007. Palliative care for patients with amyotrophic lateral sclerosis: “prepare for the worst and hope for the best”. JAMA 298: 207–16. doi: 10.1001/jama.298.2.207 [DOI] [PubMed] [Google Scholar]
- Monteleoni C., and Clark E.. 2004. Using rapid-cycle quality improvement methodology to reduce feeding tubes in patients with advanced dementia: Before and after study. BMJ 329: 491–4. doi: 10.1136/bmj.329.7464.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran B. J., Taylor M. B., and Johnson C. D.. 1990. Percutaneous endoscopic gastrostomy. British Journal of Surgery 77: 858–62. doi: 10.1002/bjs.1800770805 [DOI] [PubMed] [Google Scholar]
- Murphy L. M., and Lipman T. O.. 2003. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Archives of Internal Medicine 163: 1351–3. doi: 10.1001/archinte.163.11.1351 [DOI] [PubMed] [Google Scholar]
- Nair S., Hertan H., and Pitchumoni C. S.. 2000. Hypoalbuminemia is a poor predictor of survival after percutaneous endoscopic gastrostomy in elderly patients with dementia. American Journal of Gastroenterology 95: 133–6. doi: 10.1111/j.1572-0241.2000.01673.x [DOI] [PubMed] [Google Scholar]
- Norton B., Homer-Ward M., Donnelly M. T., Long R. G., and Holmes G. K.. 1996. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ 312: 13–6. doi: 10.1136/bmj.312.7022.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. C., Greiner M. A., Xian Y., Fonarow G. C., Olson D. M., Schwamm L. H., Bhatt D. L., Smith E. E., Maisch L., Hannah D., Lindholm B., Peterson E. D., Pencina M. J., and Hernandez A. F.. 2015. Clinical effectiveness of statin therapy after ischemic stroke: Primary results from the statin therapeutic area of the patient-centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. Circulation 132: 1404–13. doi: 10.1161/CIRCULATIONAHA.115.016183 [DOI] [PubMed] [Google Scholar]
- Odom S. R., Barone J. E., Docimo S., Bull S. M., and Jorgensson D.. 2003. Emergency department visits by demented patients with malfunctioning feeding tubes. Surgical Endoscopy and Other Interventional Techniques 17: 651–53. doi: 10.1007/s00464-002-8599-y [DOI] [PubMed] [Google Scholar]
- O'Sullivan Maillet J., Baird Schwartz D., Posthauer M. E., and Academy of Nutrition and Dietetics . 2013. Position of the Academy of Nutrition and Dietetics: Ethical and legal issues in feeding and hydration. Journal of the Academy of Nutrition and Dietetics 113: 828–33. doi: 10.1016/j.jand.2013.03.020 [DOI] [PubMed] [Google Scholar]
- Park R. H., Allison M. C., Lang J., Spence E., Morris A. J., Danesh B. J., Russell R. I., and Mills P. R.. 1992. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 304: 1406–9. doi: 10.1136/bmj.304.6839.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck A., Cohen C. E., and Mulvihill M. N.. 1990. Long-term enteral feeding of aged demented nursing home patients. Journal of the American Geriatrics Society 38: 1195–8. doi: 10.1111/j.1532-5415.1990.tb01498.x [DOI] [PubMed] [Google Scholar]
- Quill T. E. 1989. Utilization of nasogastric feeding tubes in a group of chronically ill, elderly patients in a community hospital. Archives of Internal Medicine 149: 1937–41. doi: 10.1001/archinte.1989.00390090019004 [DOI] [PubMed] [Google Scholar]
- Raguan B., Wolfovitz E., and Gil E.. 2015. Use of physical restraints in a general hospital: A cross-sectional observational study. Israel Medical Association Journal 17: 633–8. [PubMed] [Google Scholar]
- Regnard C., Leslie P., Crawford H., Matthews D., and Gibson L.. 2010. Gastrostomies in dementia: Bad practice or bad evidence? Age and Ageing 39: 282–4. doi: 10.1093/ageing/afq012 [DOI] [PubMed] [Google Scholar]
- Ribeiro Salomon A. L., and Carvalho Garbi Novaes M. R.. 2015. Outcomes of enteral nutrition for patients with advanced dementia: A systematic review. Journal of Nutrition, Health & Aging 19: 169–77. doi: 10.1007/s12603-014-0517-1 [DOI] [PubMed] [Google Scholar]
- Rimon E., Kagansky N., and Levy S.. 2005. Percutaneous endoscopic gastrostomy; Evidence of different prognosis in various patient subgroups. Age and Ageing 34: 353–57. doi: 10.1093/ageing/afi085 [DOI] [PubMed] [Google Scholar]
- Rudberg M. A., Egleston B. L., Grant M. D., and Brody J. A.. 2000. Effectiveness of feeding tubes in nursing home residents with swallowing disorders. Journal of Parenteral and Enteral Nutrition 24: 97–102. doi: 10.1177/014860710002400297 [DOI] [PubMed] [Google Scholar]
- Russell G. N., Yam P. C., Tran J., Innes P., Thomas S. D., Berry P. D., Fox M. A., Fabri B. M., Jackson M., and Weir W. I.. 1996. Gastroesophageal reflux and tracheobronchial contamination after cardiac surgery: Should a nasogastric tube be routine? Anesthesia and Analgesia 83: 228–32. doi: 10.1213/00000539-199608000-00005 [DOI] [PubMed] [Google Scholar]
- Sachs G. A., Shega J. W., and Cox-Hayley D.. 2004. Barriers to excellent end-of-life care for patients with dementia. Journal of General Internal Medicine 19: 1057–63. doi: 10.1111/j.1525-1497.2004.30329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson E. L., Candy B., and Jones L.. 2009. Enteral tube feeding for older people with advanced dementia. Cochrane Database of Systematic Reviews 2(2): CD007209. doi:10.1002/14651858.CD007209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. S., and Friedmann R.. 2006. To feed or not to feed the terminal demented patient—Is there any question? Israel Medical Association Journal 8: 507–8. [PubMed] [Google Scholar]
- Shintani S. 2013. Efficacy and ethics of artificial nutrition in patients with neurologic impairments in home care. Journal of Clinical Neuroscience 20: 220–223. doi: 10.1016/j.jocn.2012.01.054 [DOI] [PubMed] [Google Scholar]
- Tamler M. S., and Perrin J. C.. 1992. Use of a polyethylene body jacket to prevent feeding tube removal in an agitated patient with anoxic encephalopathy. American Journal of Physical Medicine and Rehabilitation 71: 236–38. doi: 10.1097/00002060-199208000-00007 [DOI] [PubMed] [Google Scholar]
- Taylor C. A., Larson D. E., Ballard D. J., Bergstrom L. R., Silverstein M. D., Zinsmeister A. R., and DiMagno E. P.. 1992. Predictors of outcome after percutaneous endoscopic gastrostomy: A community-based study. Mayo Clinic Proceedings 67: 1042–49. doi: 10.1016/S0025-6196(12)61118-5 [DOI] [PubMed] [Google Scholar]
- Teno J. M., Gozalo P. L., Mitchell S. L., Kuo S., Rhodes R. L., Bynum J. P., and Mor V.. 2012. Does feeding tube insertion and its timing improve survival? Journal of the American Geriatrics Society 60: 1918–21. doi: 10.1111/j.1532-5415.2012.04148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teno J. M., Mitchell S. L., Kuo S. K., Gozalo P. L., Rhodes R. L., Lima J. C., and Mor V.. 2011. Decision-making and outcomes of feeding tube insertion: A five-state study. Journal of the American Geriatrics Society 59: 881–86. doi: 10.1111/j.1532-5415.2011.03385.x [DOI] [PMC free article] [PubMed] [Google Scholar]