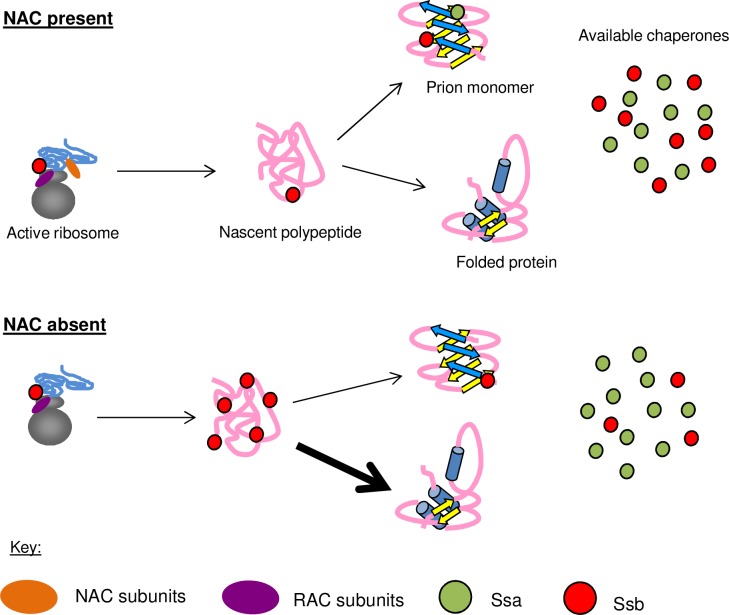
Fig 8. NAC subunits affect the yeast chaperone network by altering chaperone pools.
In the presence of the NAC, RAC-Ssb receives nascent polypeptides from the NAC and assists with their folding. Both Ssa and Ssb bind to aggregated Sup35 and affect its ability to form and propagate the [PSI+] prion. When NAC subunits are deleted, Rac-Ssb becomes the first complete chaperone system to interact with nascent polypeptides. The unfolded state of these proteins requires more extensive interaction with RAC-Ssb, thereby sequestering cytosolic Ssb to the ribosome. Thus, less cytosolic Ssb is available to bind to monomeric or aggregated Sup35. This would lead to a relative increase in Ssa1 binding to Sup35 aggregates, which inhibits efficient monomer joining and reduces the ability of Hsp104 to cure the prion.