Abstract
Tropical forests with a low human population and absence of large-scale deforestation provide unique opportunities to study successful conservation strategies, which should be based on adequate monitoring tools. This study explored the conservation status of a large predator, the jaguar, considered an indicator of the maintenance of how well ecological processes are maintained. We implemented an original integrative approach, exploring successive ecosystem status proxies, from habitats and responses to threats of predators and their prey, to canopy structure and forest biomass. Niche modeling allowed identification of more suitable habitats, significantly related to canopy height and forest biomass. Capture/recapture methods showed that jaguar density was higher in habitats identified as more suitable by the niche model. Surveys of ungulates, large rodents and birds also showed higher density where jaguars were more abundant. Although jaguar density does not allow early detection of overall vertebrate community collapse, a decrease in the abundance of large terrestrial birds was noted as good first evidence of disturbance. The most promising tool comes from easily acquired LiDAR data and radar images: a decrease in canopy roughness was closely associated with the disturbance of forests and associated decreasing vertebrate biomass. This mixed approach, focusing on an apex predator, ecological modeling and remote-sensing information, not only helps detect early population declines in large mammals, but is also useful to discuss the relevance of large predators as indicators and the efficiency of conservation measures. It can also be easily extrapolated and adapted in a timely manner, since important open-source data are increasingly available and relevant for large-scale and real-time monitoring of biodiversity.
Introduction
Despite continuous and increasing local efforts and political initiatives, habitats and species continue to decline worldwide, and projections remain pessimistic [1, 2]. However, long before spectacular deforestation [3], human pressure may result in "empty forests" [4]. Impacts of defaunation are cryptic and pernicious [5]. Population decreases of species with important ecological roles modify ecosystem engineering, seed dispersal and plant recruitment [6–8], as well as community structure [9]. Beyond species loss and their associated functional roles, the loss of ecological interactions may also amplify the consequences of losing ecological services [10].
The pace of global forest habitat loss in South America, most particularly Amazonia, is also on the rise. Amazonian forest ecosystems, like other tropical forests, are also threatened by large-scale defaunation [11]. This forest loss nevertheless remains much lower in the Guiana Shield: Guyana, Suriname, French Guiana and the Brazilian state of Amapá [3,12]. This region is the largest contiguous exposed Precambrian rock in South America, covering more than 2 million km2 of northeast Amazonia [13]. This historical absence of large-scale deforestation makes this region the largest repository of tropical forest vegetation on Precambrian terrain in the world [14]. The region is an area of major importance for conservation of several large terrestrial mammals, including peccaries (Tayassu pecari and Pecari tajacu), Brazilian tapir (Tapirus terrestris) [15], giant and neotropical otters (Pteronura brasiliensis and Lontra longicaudis) [16, 17], and jaguar (Panthera onca) [18]. Threats to their survival are nevertheless present and growing in the region. However, much more than agriculture issues and fires, as elsewhere in Amazonia, gold mining is the main threat in the region [19–21], followed by increasing human population growth, unregulated hunting and widespread unsustainable animal harvesting [22,23], all of which threaten the future of vertebrate communities [24].
Carnivores are widely recognized indicators of forest species decline, and they focus attention on broad conservation approaches. Recently, many species have been studied using the latest niche modeling tools to improve knowledge of ecological requirements and conservation opportunities [17,25,26]. Nevertheless, approaches considering the conservation status of both predator and prey species, the relations between their respective status and the prospectives for the future are scarce and difficult to implement. The jaguar (P. onca) is the largest Neotropical felid in the Americas, considered to be "near threatened" [27], with decreasing populations mainly caused by habitat loss and currently occupying less than 50% of their historical range [18]. The major threats to jaguars in Amazonia are habitat loss, habitat fragmentation and hunting of both jaguars and their prey. The jaguar is not only a relevant umbrella species for wide biodiversity conservation, but can be considered a good indicator of healthy trophic cascades [5,28,29]. The resources required for relevant assessment of population density [30] may nevertheless be beyond the means of most conservation initiatives. Alternatively, the community of large vertebrates, which may be important prey species for large felids, is a good surrogate to determine the human footprint [24], although for many species a relevant assessment of abundance also requires extensive field work [31]. In contrast, recent developments in remote sensing and aerial imaging have provided new approaches to detect habitat disturbance [32–36] and may help identify depleted and degraded areas and/or areas facing a high risk of population collapse with changes on target species communities and ecosystem functions [37].
Therefore, this study explored how predator and prey species as well as habitat structure are interlinked, and more specifically:
Jaguar distribution was assessed within the so-called highest-priority French Guiana and Amapá Jaguar Conservation Unit (JCU) [18] where ecological processes are assumed to have remained stable. We used species distribution modeling (SDM) to identify more favorable, appropriate environmental conditions for jaguars at the country scale, expected to be associated with a higher likelihood of long-term persistence [25].
Classical camera-trap survey procedures and capture-recapture models [38] were used to assess jaguar density on four sites and to test how predicted environmental conditions are related to field-measured densities.
Proxies of jaguar and prey status and key habitats are suggested.
Broader discussion is opened on the relevance of felids as proxy for habitat and fauna community status, and how more rapidly acquired field information, associated with open-source data, can be obtained to inform on large vertebrate populations and to provide opportunities for assessment of species conservation status in areas where field constraints are important.
Methods
Study area
This study took place in French Guiana, a French administrative unit covering ~84,000 km2 located on the northern part of South America, on the Guiana Shield. The Guiana Shield is one of the largest pristine Neotropical rainforest blocks and a floristically distinctive province compared to the Amazonian basin [39]. Eighty percent of French Guiana is covered by upland moist forests implanted on generally well-drained clayic ferralic soils (i.e., Ferralsol and Acrisol; [40]) over altitudes of 0–600 m. Canopy reflectance has defined five main forest types in terms of structure: low dense forests, high forests with regular or disrupted canopy, mixed high and open forests, and Euterpe palm forests [41]. Tree species composition varies depending on the relief: very diversified on the all-slope reliefs, the forest is generally dominated by Lecythidaceae on the northern hilly multiconvex reliefs, the Leguminosae-Caesalpinioideae on the central tablelands and Burseraceae on the southern inland plains [42]. The alluvial coastal plain is covered by marsh forests, savannas, transition forests, and herbaceous swamps and is rather narrow on this part of the Guiana shield [43]. Compared to other Neotropical countries, the forest conservation status of eastern Venezuela, Guyana, Suriname, French Guiana and the Brazilian states of Amapá and Pará is still rather favorable. French Guiana benefits from an extensive network of protected areas including five nature reserves, located in patches in the northern half of the country, and a national park in the south, for a total protected area of 23,000 km2 (>25% of the country).
SDM modeling of jaguars: identification of more suitable habitats
Volunteer participation of naturalist networks (environmental NGOs, a public database, scientists) allowed us to record 302 recent sightings (2008–2014) of jaguars on different 0.5 × 0.5-km units. We used a maximum entropy procedure (MaxEnt 3.3.3k, [44]) to estimate the probability distribution of the maximum entropy of each environmental variable across the study area. This analysis has a recognized effectiveness in processing presence-only data and small data sets [45]. To control the likely geographic bias of sighting distribution, the model was forced to use environmental layers restricted to the sampling areas during the learning stage [46]. Predicted areas of occurrence were then projected at the country scale. The model was run using 75% of records for training and the remaining 25% for testing. Five thousand iterations were used with a bootstrap replicate strategy. A 1.0 × 10−5 convergence threshold, logistic output format and linear/quadratic regularization values were set. The following predictive environmental data were used to investigate the occurrence of species and to predict more suitable habitats: pluviometry [47]; mean altitude and range of altitude within the grid [48]; vegetation types defined with low spatial resolution remote-sensing data [41]; biogeographic units [49]; and the human footprint representing the distribution and strength of pressures on natural habitats [24]. The addition of environmental data (pluviometry, altitudes, biogeography) and more local idiosyncratic factors (vegetation types, footprint) may help predict broad spatial structures while bionomic variables may tend to present finer grained spatial patterns [50]. The models were interpreted with the AUC test [51]. However, because the AUC test could lead to misinterpretation of model accuracy [52], the null model hypothesis [53] was also used to test the performance of the predictions. We generated 99 random distributions and considered the 95th AUC value as the upper limit of the 95% CI of the AUC. Then, as soon as the AUC value of one species was higher than this 95th ranked AUC, the accuracy of the SDM was significantly higher than expected by chance alone with p<0.05.
Camera trapping: density assessment
Four sites were surveyed in the north and center of French Guiana, in terra-firme highland forests. From west to east, the sites were (1) The Montagne de Fer ("MdeFer", center of the area: 05°20' N, 52°32' W), (2) Counami forest ("Counami", center of the site: 05°18'N, 53°05 W), (3) Montagne de Kaw ("MdeKaw", center area: 04°35' N, 52°20' W), and (4) The Nouragues Nature reserve ("Nouragues", center area: 04°10' N, 52°40' W) (Fig 1). The first two sites are dominated by dense, regular canopy forest [41] and were formerly logged forests. Low-impact logging practices are implemented in these areas, with fewer than five trees extracted per hectare [54] and fully controlled logging activities. The third site was a nearly pristine forest area, partly protected by two nature reserves, Kaw-Roura National Nature Reserve, and Trésor Regional Nature Reserve. The fourth site is a pristine and fully protected area, dominated by dense and regular canopy forest. Sites (1) and (2) did not require specific permission for implementing the studies. Studies on sites (3) and (4) were conducted with the permission formalized by the agreement signed between the National Forest Agency, the NGO AGEP (managers of the Nature Reserve), and the NGO Kwata (in charge of the study).
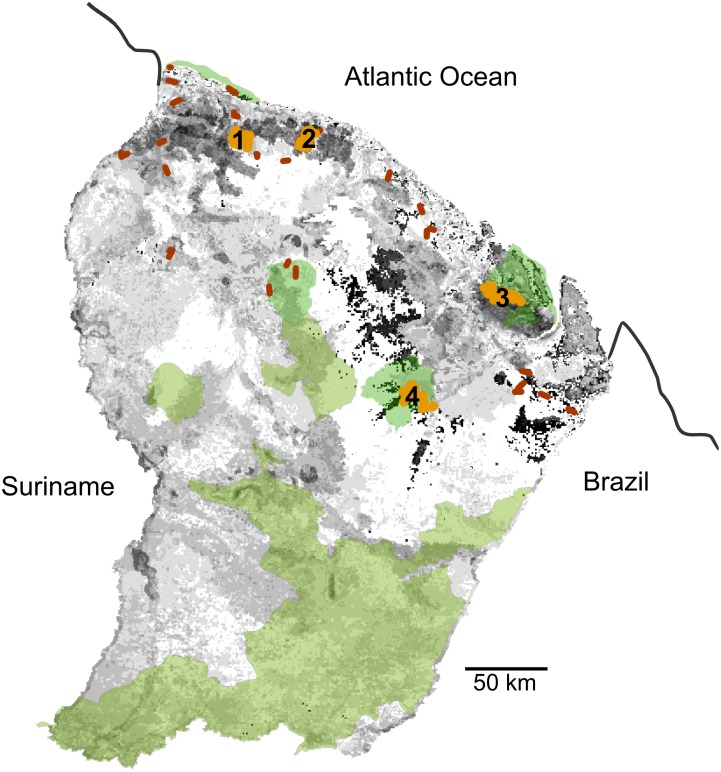
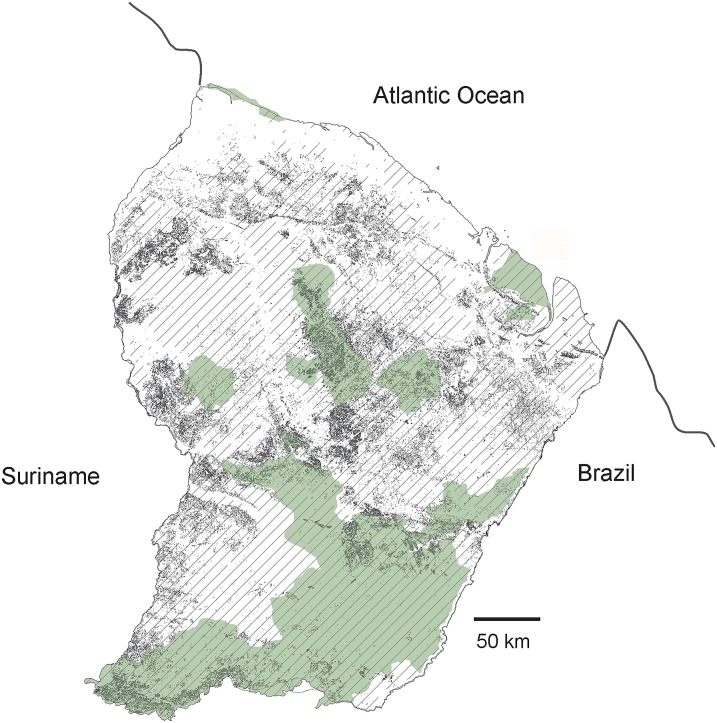
Fig 1. French Guiana study sites, with the protected areas.
Protected areas: nature reserves and national parks, in green. Locations of the sites where camera-trap surveys were implemented (1, Montagne de Fer; 2, Counami; 3, Montagne de Kaw; 4, Nouragues) in light brown; locations of the areas where line-transects were implemented in dark brown; and jaguar habitats from less favorable (light grey) to more favorable (dark grey).
Surveys were implemented in 2007, 2008, 2009 and 2010 (sites 1, 2, 3 and 4, respectively) during the dry season, from September to December. On each site, 16–18 stations were spaced 2–3 km apart (MdeFer: m = 2.2 ± 0.3; Counami: m = 2.2 ± 0.4, MdeKaw: m = 2.5 ± 0.5, Nouragues: m = 2.4 ± 0.9) with two cameras per station (DeerCam and CamTrakker argentic models for Montagne de Fer, Counami and Montagne de Kaw, and Reconyx 500 for the Nouragues reserve). Traps were in place for 3 months, a reasonable duration to fit with the population closure requirement for CMR models [38]. The surveys included 1,656 station/nights for MdeFer, 1,690 on Counami, 1,530 on MdeKaw, and 1,870 for Nouragues. Data analysis followed classical methodologies, including animal identification based on skin spots, assessment of theoretical population size with capture/recapture methods (CAPTURE, [55]) and assessment of effective study area using recapture distances. A first classical buffer of ½ MMDM (half of the mean maximum distance moved by individuals photographed on separate capture occasions [38]) was used. Half the MMDM may nevertheless result in overestimation of abundance, and a MMDM buffer is recommended [30].
Abundance of prey species
To investigate the relations between the habitats, the occurrence of the jaguar and the prey community, we explored the abundance of selected jaguar prey species [56,57], including two rodents (Myoprocta acouchy and Dasyprocta leporina), three Artiodactyla (Mazama nemoviraga, Mazama americana, and P. tajacu), and two large frugivorous birds (Crax alector and Psophia crepitans) on a wide range of sites. Abundance was measured with the line transect method on 30 sites, with a mean area covering 97 ± 2.1 km expected to provide a stabilized assessment of abundance [31] (Fig 1). The 30 sites were distributed in most terra firme forest landscape units [40,49], in order to encompass regional particularities related to given forest habitats [58]. Additionnal information on survey sites are provided in S1 Table. The correlations were tested between species abundance (expressed with a kilometric index [KI], i.e., the number of contacts, or individuals in case of gregarious species, per kilometer), the biomass index (KI × mean adult weight, with weights derived from [59] and (i) the predicted occurrence of the jaguar (i.e., the suitability score derived from SDM at the survey site), (ii) the human footprint [24] and (iii) the habitat structure defined with canopy heights and above-ground biomass (AGB) (see below). To assess the area required to target stabilized abundance, we considered the correlation between the asymptotic abundance value (sampling area > 100 km [24]), and abundance assessed with a smaller sampling effort.
Habitat structure, jaguars and prey
On these 30 sites, four data sources were used to describe habitats: i) maximum canopy height from a full waveform space-borne LiDAR; ii) canopy height as well as roughness and terrain data from the LiDAR waveform length; iii) AGB; and (iv) geology. The LiDAR data were acquired from the Geoscience Laser Altimeter System (GLAS) onboard the ICE, Cloud and land Elevation Satellite (ICESat). The GLAS sensor, which orbited Earth from 2003 to 2009, acquired data using a 1064-nm laser with a nominal footprint on the earth’s surface of approximately 65 m and a distance of 172 m between each footprint. Each waveform contains data on the time variations in the intensity of the energy returned from each laser pulse, thus providing information on the vertical structure of the canopy. Several variables related to vegetation structure can be extracted from the GLAS waveforms [60–63]. However, in this study only the waveform extent, which corresponds to the distance between the signal’s beginning and end, was used. The waveform extent provides information on the combination of vegetation height and the effect of topographic slope [60]. Canopy height estimation resulted from [64], these estimations produced a precision on the canopy height estimation of 3.6 m. Finally, a 3 × 3-pixel spatial standard deviation of terrain data derived from the Shuttle Radar Topography Mission (SRTM) Digital Elevation Model (DEM) were used as a proxy for canopy roughness. AGB estimates stem from a spatial predictive model [65]. This prediction map is based on the inventories of 2507 field plots in undisturbed rainforest (0.4–0.5 ha) distributed over the entire region. The model was developed by kriging-regression in order to include spatial and environmental effects on AGB. The relationship between the niche model, the abundance of prey species, canopy structure, terrain roughness, and AGB was studied using random forests [66]. For the geology, a geological substratum map produced by the French Geological Survey [67] was used. The map was simplified in order to retain only the five largest rock formations: recent sediments, volcanic sedimentary rock, granites, gabbros and gneiss. This simplification was required so that each geology class could be sampled with satisfactory accuracy.
Results
Distribution modeling and density for the jaguar
Predicted jaguar occurrence distribution is highly reliable (area under the curve, AUC = 0.832), and the reliability of the null hypothesis that the accuracy of the model was significantly higher than expected by chance was greater than 95% (p<0.05). The jackknife test on both test gain and the AUC showed a major contribution of rain and biogeography in the explanation of the distribution. Fig 1 shows the areas where the most favorable conditions were identified with the MaxEnt model. These areas include many habitats in the northern part of the country, in the so-called low joint valleys and complex multiconvex landscapes, and more in the south on low plateaus and mountains (sensu, [42]). On the four sites where density was measured, the number of jaguars identified ranged from six to nine. Depending on the sites and species, CAPTURE identified either Mo (null estimator) or Mh (jackknife estimator) as the best population estimators and calculated eight to ten animals (Table 1). Survey areas calculated on the basis of ½ MMDM (animals captured at more than one camera station, from MdeFer: three (Counami, MdeKaw) to eight individuals (MdeFer); Counami: three individuals; MdeKaw: three individuals; Nouragues: five individuals), were 194, 246, 275 and 229 km2 for MdeFer, Counami, MdeKaw and Nouragues, respectively. Associated calculated density values ranged from 2.9 adults/100 km2 at MdeKaw to 5.1 adults/100 km2 at MdeFer. The survey areas calculated on the basis of MMDM and associated densities ranged from 562 km2 and 1.4 adults/100 km (MdeKaw) to 405 km2 and 2.5 adults/100 km2 (MdeFer). Despite the substantial differences in the number of recorded animals (Table 1), mean densities did not statistically differ between the four sites, and consequently were not influenced by the Human Footprint Index.
Table 1. Results of four camera-trapping surveys in French Guiana for jaguars.
| Nb of animals identified / Nb of animals × 100 km2* | Calculated population | Mean D max (km) | Surveyed area / density (for 100 km2 (1/2 MMDM) | Surveyed area /density for 100 km2 (MMDM) | |
|---|---|---|---|---|---|
| 1. Montagne de Fer | 9 / 14.0 | 10 [SE = 3.0] | 6.6 | 194 km2 / 5.1 ind.(SE = 3.6–6.7) | 405 km2 / 2.5 ind. (SE = 1.7–3.2) |
| 2. Counami | 6 / 9.0 | 8 [SE = 2.1] | 7.8 | 246 km2 / 3.3 ind. (SE = 2.8–4.1) | 530 km2 / 1.5 ind. (SE = 1.3–1.9) |
| 3. Montagne de Kaw | 6 / 6.7 | 8 [SE = 1.9] | 6.6 | 275 km2 / 2.9 ind. (SE = 2.2–3.6) | 562 km2 / 1.4 ind. (SE = 1.1–1.8) |
| 4. Nouragues | 9 / 10.2 | 10 [SE = 2.5] | 7.1 | 229 km2 / 4.4 ind. (SE = 3.3–5.5) | 507 km2 / 2.0 ind. (SE = 0.15–2.5) |
* as defined by the minimum convex polygon size
Habitats and prey communities
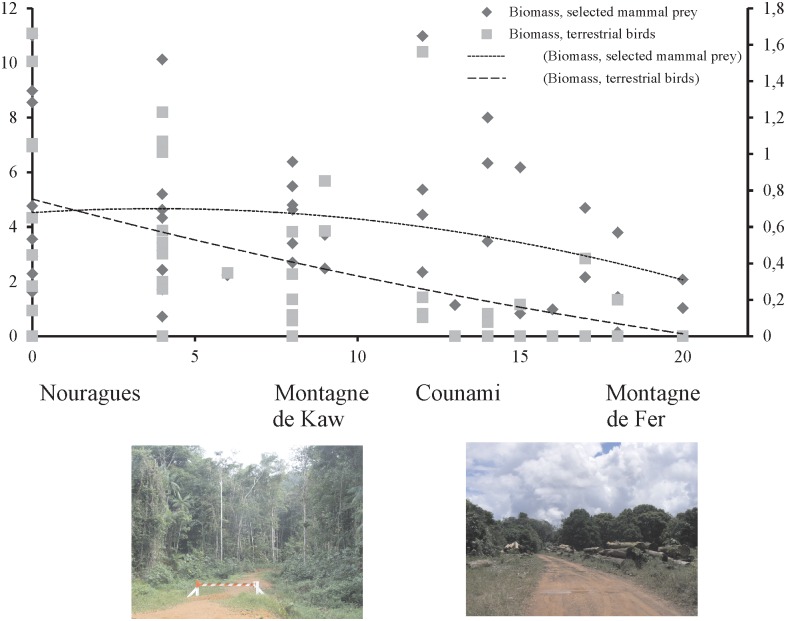
Of the 30 sites, the cumulated abundance of the five target mammal species (M. acouchy, D. leporina, M. nemoviraga, M. americana, P. tajacu) and of the two birds (C. alector and P. crepitans) were negatively correlated with the human footprint (p(uncorr.) = 0.03, r = −0.31 and p(uncorr.)<000.1 r = −0.63, respectively). The biomass value of birds was also negatively correlated with the human footprint (r = −0.63, p = 0.0002), the biomass of mammals was also negatively correlated to the footprint, although the relation was not significant (r = −0.32, p = 0.07) (Fig 2). Although concomitantly measured on four sites only, prey was more abundant where higher densities of jaguars were recorded, but neither biomass nor abundance was correlated with the jaguar’s predicted favorability index.
Fig 2. Biomass Index (kg-1 × km of transect) of a selected set of terrestrial birds and mammals, according to the Human Footprint Index, and associated correlation curves.
The four study sites for jaguar density assessment are given on the index scale, according to their mean value. The two pictures show sites with a 5–10 Index value (left) and a 15–20 Index value (right).
Early indicators of population status and collapse?
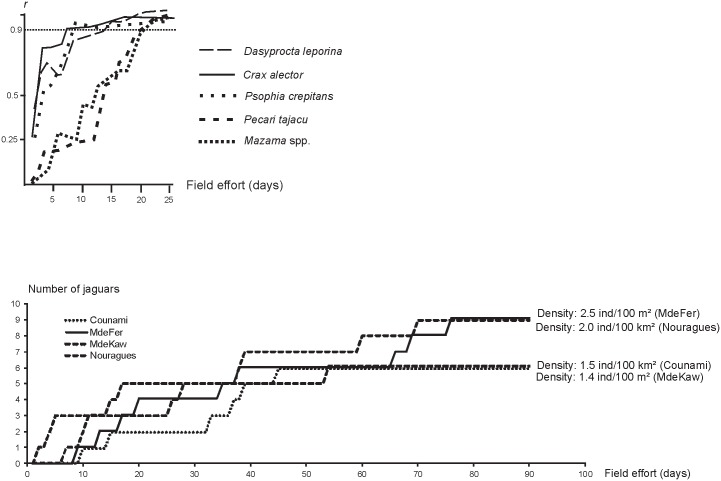
The accumulation curves of new jaguars recorded in each survey area stabilized after considerable field work: 50 days (1000 traps/night) on the sites with lower density, and 80 days (1600 traps/night) for the two sites with higher density values (Fig 3). Field work for assessment of high vertebrate abundance shows that a survey longer than 20 days (cumulative distance greater than 80 km of linear transect) is necessary to relevantly assess the abundance of ungulates; the field work required could be shorter for large birds (50–60 km of linear transects, i.e., 8–10 days) (Fig 3).
Fig 3. Field surveys required to assess abundance of ungulates and two frugivorous birds, based on 40 surveys (up), and the number of jaguars based on four study areas (down).
Top: the r coefficient correlation shows the correlation between the stabilized abundance (sampling effort > 100 km) and abundance assessed with a lesser sampling effort. The correlation is derived from 40 surveys.
Canopy roughness was negatively correlated with the human footprint and was higher in more pristine habitats (r = −0.66, p(uncorr.)<0.001). The AGB model cannot integrate the effect of human disturbance: remote-sensing data as well as field data on disturbed plots were not included in the analysis [65]. Canopy roughness was correlated with the abundance and biomass of large terrestrial birds (r = 0.73, p(uncorr.)<0.001 and r = 0.74, p(uncorr.)<0.001, respectively), although relations between AGB and large vertebrate abundance were not significant. Finally, a random forest classification showed that the jaguar distribution on one hand and canopy roughness and geology on the other hand, were significantly correlated (r = 0.61, p(uncorr.)<0.01). Adding more information in addition to the canopy structure, such as maximum canopy height from GLAS, GLAS waveform extent, terrain roughness, geology and AGB, the strength of the relation between the niche model and these variables increased (r = 0.71, p(uncorr.)<0.01).
Discussion
In addition to direct habitat loss that can be monitored via direct imaging or with more precise tools (e.g., biomass and carbon measurements [68]), much more cryptic threats such as hunting and its cascading effects comprise the main threat in tropical forests [4], requiring adequate and early indicators. We focused on the region of the Guianas, in northern South America, confronted with a dual challenge: (i) the need to reinforce its recognition as an area of major importance for long-term conservation of forest species and (ii) the need to mitigate increasing human population growth, with its cascades of pressures on forest ecosystems. We developed our approach on predator, prey and habitats, and expect to detect early signs of population collapse, before shifting to empty forests.
Species distribution modeling and jaguar density
Jaguars were recorded in most of the country, and no large area of local extinction was evidenced. Niche modeling identified some habitats as more favorable, on the northeastern and center relief landscape belts. These favorable regions were previously detected in the wider-scale approach [25] and include "complex multiconvex landscapes," "slopes," "low plateaus" and "mountains" (sensu, [42]). A previous study showed that the α diversity of large vertebrates, expected to be prey for predators, was low on those landscapes [58]. But although only a few vertebrate species showed a marked preference for those areas, the red brocket deer (M. americana), the collared peccary (P. tajacu) and the red agouti (D. leporina) were the only species with a clear preference for the complex multiconvex landscapes, low plateaus and mountains of these environments [58]: they may be important resources for the jaguar in these landscapes.
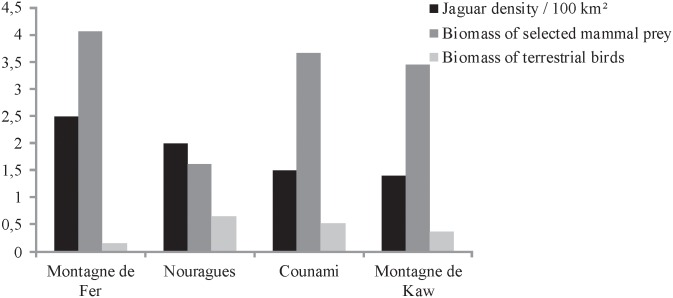
Regarding density, assessments were roughly within the ranges reported in the central and south regions of South America [69] and are expected to reflect healthy populations. Comparisons with other sites should nevertheless be interpreted cautiously, since these data from French Guiana are the first available for the northern Amazon region. At a more local scale, although not significant, differences in felid density were noted between the four sites. The two sites with higher jaguar density were the least disturbed areas: the Nouragues Nature Reserve and the Montagne de Fer, which is a formerly logged area. The network of ancient logging tracks at Montagne de Fer could also have slightly biased the results. In jaguars, the higher tolerance of males to human-modified landscapes, including the attractiveness of tracks [70], increased the capture rate and may have slightly overestimated jaguar density despite the correction by the capture-recapture models. Nevertheless, the rough number of animals detected, weighted by the size of the survey area, remained higher at Nouragues and Montagne de Fer, reinforcing the idea of a large population on the latter site (Fig 4).
Fig 4. Fauna communities on the four study sites with implementation of both camera-trapping and linear transects: jaguar density, biomass index of mammal prey, biomass index of terrestrial bird prey.
The performance of the model, further confirmed by field-measured density, also highlighted the most important areas for the future of populations, directly related to conservation issues. Although the southern areas benefit from National Park protection, the favorable habitats located in the north of the country are less covered by protected areas (Fig 1) and therefore require that particular attention be paid to these areas for future populations.
Prey and forest structure provide better information on jaguar habitats
Ungulates (M. nemoviraga, M. americana, P. tajacu) and large frugivorous terrestrial birds (C. alector, P. crepitans) are expected to be key resources for large felids [71]. When compared to other forest surveys in Amazonia [72–75], the abundance of these species measured in French Guiana confirms the idea of the overall good status of forest ecosystems. However, the general pattern observed of more abundant prey on more suitable habitats for jaguar is not significant. This suggests that the jaguar’s diet may be highly diverse and depend on a wider range of prey species than considered here, including smaller species. Also, we previously evidenced that some opportunistic species, such as agouti, collared peccary and tapirs, may maintain or even take demographic advantage in old logged forests [24,76], explaining the high density observed at Montagne de Fer and helping ensure the future of jaguar populations on those managed forests.
Negative responses of prey abundances to Human Footprint Index (either highly significant in the case of terrestrial birds, or close to significance in the case of terrestrial mammals), suggesting that the mega vertebrate community can persist in selectively logged forests [77], but unmanaged hunting pressure could result in substantial population declines and threatened prey and predators in a cascading effect. Twenty-five percent of the country is above a threshold value, which may indicate that populations of ungulates, large rodents and large terrestrial birds are beginning to collapse [24]. Long before deforestation and forest habitat loss, estimated over the last decade (2000–2012) at less than 20,000 ha, i.e., less than 0.2% of the territory (derived from [3]), overhunting is the main threat for these species. Assessment of hunting sustainability has not been investigated in French Guiana for Artiodactyla, but monitoring of primate hunting has shown unsustainable harvests in many settlements, including in local and traditional communities [23]. Similar results were obtained for tapirs [78]. Given the near absence of hunting management in French Guiana, hunters’ access to large forest areas via logging tracks and hunting pressure exerted by several tens of thousands of gold miners [19,21], the greater threat to jaguar survival could likely be the negative trends of prey abundance due to hunting.
Regional conservation issues
With wide-ranging species such as large carnivores, together with the range-wide conservation network [79], the identification of refugia and lower-quality habitat are necessary for long-term conservation planning, which may require fine-management-scale units [80]. Human-use areas are also important habitats for connectivity and dispersal of jaguars between core protected areas [81].
Setting priorities for conservation in largely undisturbed regions such as the Guianas may nevertheless differ from those commonly applied to highly disturbed and human-dominated regions [82]. The Amapa–French Guiana Jaguar Conservation Unit of has been listed as the highest priority, with substantial opportunities for large-scale dispersals due to lack of fragmentation with highly conserved Southern forest blocks, and an estimated population size of more than 500 animals [83]. Although this very open and unfragmented population precludes adequate population viability analysis [84], many parts of the Guianas still provide unique opportunities to conserve species with large ecologic and spatial requirements and are expected to ensure its future. The first lesson of this long-term survey of jaguars and their prey is the good status of conservation in French Guiana and likely the entire eastern part of the Guiana shield, with no evidence of widespread jaguar depletion. Governmental management of forests, selective and reduced-impact logging techniques [85] and the network of protected areas have safeguarded a satisfactory status of forest habitats, associated species and trophic cascades. Although the SDM approach identified less favorable habitats (Fig 1), these areas are unfragmented, far from anthropic threats and connect areas of importance. They consequently should not be considered as "ecological traps" (sensu [80]) but as landscape connectivity between preferred areas.
Regarding habitat loss, gold mining activities [15] are responsible for an annual loss of 2,000 hectares of forest habitat, and the recent alarming increase of deforestation due to the demand for gold has been spotlighted [17]. However, long before deforestation, defaunation and empty forests threaten tropical ecosystems. The main concern in the Amapá and French Guiana Jaguar Conservation Unit is overhunting of prey. A decades-long lack of hunting regulation, widespread and cryptic harvests of wild species by goldminers, and demographic expansion of local communities with little access to alternative resources result in silent shifts of rich forests and empty areas. Similar to other Amazonian regions spared by large-scale deforestation [23,86], insufficient management of hunting is likely the most significant threat for ungulates, large birds and the top predators.
Beyond the Guiana jaguars and canopy: new tools and opportunities for wildlife conservation
Conservation initiatives require adequate proxies to detect early and cryptic population collapses and often suffer from lack of data and limited access to large field inventories. The relevance of the proxies is based on four assumptions: (i) comprehensive sets of input data, (ii) relevant explanatory variables (i.e., environmental descriptors), (iii) good indicator species and (iv) adequate modeling procedures. This raises the question of the target species and the relevance of charismatic species (i.e., species that will draw more attention from nonprofessional recorders) as proxies. Large carnivores and apex predators are widely accepted as indicators and keystone species, and furthermore are often threatened [87,88]. However, the jaguar has recognized opportunistic behavior and diet plasticity with a wide range of prey consumed, including small species [89] that may benefit from forest cover changes [90], for which forest managers may miss early signs of population disturbance and delay detectable responses, are consequently insufficient early indicators of forest and animal community disturbances, at least at the relatively low extent and strength levels observed in the Guianas. We can concur with previous research [91] and assume that large carnivores may not be locally relevant indicators for local and/or early changes in forest animal communities encountering rather low and cryptic threats.
The abundance of some prey species may be a better proxy for the threat to jaguars. However, field work for assessing large vertebrate density and abundance in forest habitats is also important and could delay the ability to detect a population’s collapse. For instance, we previously showed that a cumulative distance greater than 100 km of linear transect could be necessary to relevantly assess the abundance of ungulates [24]: regular monitoring in protected areas and/or in managed forest areas may be limited. The field work required could be shorter for large birds (50–60 km of linear transects, i.e., 8–10 days) (Fig 3). Focusing on these species only, which are also the first to collapse [24], could provide a relevant indicator.
Large sets of open and/or shared data [92,93] allow modeling, extrapolation and assessment of population trends at uncovered resolution and open new avenues for conservation. Increasing enthusiasm for participative science, or "public participation in scientific research," is accepted as a win–win model [94], not only because it results in increased knowledge of biodiversity issues and of the participants’ local environments, but also because engagements in science are expected to change attitudes and environmental behavior [95,96]. In the present case, more than 75% of the jaguar records come from non-scientist recorders, obviously facilitated by the charismatic value of the species. Once such data sets are properly analyzed, they may successfully contribute to biodiversity monitoring. Species distribution models are increasingly used for conservation science [97] and can efficiently contribute to this task, transforming citizen data into identification of areas acting as sources and corridors, and providing original conductance and/or resistance values for landscape connectivity modeling [76].
Far more promising are the data derived from remote-sensing and radar/LiDAR analysis of forest structure. Here we showed that information based on the latest remote-sensing and plane-imaging techniques can detect fine-scale damage to the canopy cover and may inform on the status of associated fauna communities. AGB, canopy height and soil roughness informed on jaguar habitat and showed a clear relation to the predicted favorability and may highight, when surimposed with the Human Footprint index driving abundances of prey, areas of importance for long-term maintenance of ecological dynamics (Fig 5). Complex canopy structure, roughness and high AGB levels were correlated with a higher predicted occurrence of jaguar. In Chile, mesocarnivores also responded to fine-grain habitat structure attributes, identified from LiDAR, much more than to habitat, landscapes and vegetation classifications [98]. Although biotic relations between forest structure, phenology, vertebrate communities and ultimately predators will require further investigation, the high level of confidence of the relations observed between these factors already suggests that this tool will help identify key areas for conservation. Remote-sensing measurements could help detect the scale at which animals discriminate habitat characteristics, as well as the scale at which conservation priorities and plans must be implemented. Compared to categorical classifications, more gradient-based analyses of landscape architecture will yield more realistic representations of ecological heterogeneity and better predict species responses and needs [99].
Fig 5. French Guiana more favorable habitats of prey.
Protected areas: nature reserves and national parks, in green. Hatchings: area with Human Footprint Index < 15 (thresold value allowing maintenance of the abundance of most of prey species). Dark grey: forest areas with canopy roughness > 12, favoring higher biomass of prey.
Spatial and aerial images have already shown their usefulness in detecting large- and fine-scale habitat disturbance [100]. We also show here that LiDAR provides a relevant indication of forest disturbance and may easily inform on the status of habitats and associated fauna communities. Increasingly efficient tools, based on remote sensing and innovative spatial analysis methods (e.g., [101]), are being developed and will soon be operational, e.g., Sentinel 1 (radar) and Sentinel 2 (optical) satellite constellations from the European Space Agency. They will be useful to monitor tropical rainforest habitats. At this time, different techniques can be applied to obtain data dependently on the degree of precision required. In the present study, LiDAR has shown that it can estimate canopy roughness at a large scale; other techniques such as FOTO (FOurier Textural Ordination) [102] should also be explored. Furthermore, more and more open-source data are available at wide geographic scales [36]. Characterizing structure and functioning habitats to identify threats on flora and fauna are key points in the global estimation of ecosystems services in general, and of biodiversity in particular. Remote sensing provides a wide panel of investigative tools to monitor habitats and help decision makers in their conservation policies [103], and they can be particularly useful in remote forest areas as early indicators of the first signs of habitat damage and subsequent threats to animal communities.
Supporting Information
(XLSX)
Acknowledgments
The camera-trapping surveys were funded by the Wildlife Conservation Society and the SPECIES project, the WWF Network, European Funds (FEDER), FFEM, the DGIS and the French Ministry of Higher Education and Research. Field surveys for prey were funded by Diren Guyane, DEAL Guyane, ONF Guyane, and the Réserve Naturelle Nationale de la Trinité. We are grateful to Leo Maffei (WCS) for his advice and assistance in the field for the first camera trapping session in French Guiana. We acknowledge the Nouragues Nature Reserve, the CNRS Guyane, and the DEAL Guyane, for field support during the Nouragues trap session.
Data Availability
Data are from the current study and are provided as Supporting Information.
Funding Statement
The camera-trapping surveys were funded by the Wildlife Conservation Society and the SPECIES project, the WWF Network, European Funds (FEDER), FFEM, the DGIS and the French Ministry of Higher Education and Research. Field surveys for prey were funded by Diren Guyane, DEAL Guyane, ONF Guyane, and the Réserve Naturelle Nationale de la Trinité.
References
- 1.Butchart SHM, Walpole M, Collen B, van Strien A, Scharlemann JPW, Rea A, et al. Global Biodiversity: Indicators of Recent Declines. Science 2010; 328: 1164–1168 10.1126/science.1187512 [DOI] [PubMed] [Google Scholar]
- 2.Tittensor DP, Walpole M, Hill SLL, Boyce DG, Britten GL, Burgess ND, et al. 2014. A mid-term analysis of progress toward international biodiversity targets. Science 2014; 346: 241–244. 10.1126/science.1257484 [DOI] [PubMed] [Google Scholar]
- 3.Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, et al. High-Resolution Global Maps of 21st-Century forest cover change. Science 2013; 342: 850–853. 10.1126/science.1244693 [DOI] [PubMed] [Google Scholar]
- 4.Wilkie DS, Bennett EL, Peres CA, Cunningham AA. The empty forest revisited. Annals New York Academy of Sciences 2011; 1223: 120–128. [DOI] [PubMed] [Google Scholar]
- 5.Jorge MLSP, Galetti M, Ribeiro MC, Ferraz KMPMB. Mammal defaunation as surrogate of trophic cascades in a biodiversity hotspot. Biological Conservation 2013; 163: 4–57. [Google Scholar]
- 6.Nunez-Iturri G, Olsson O, Howe HF. Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biological Conservation 2008; 141: 1536–1546. [Google Scholar]
- 7.Beck H, Snodgrass JW, Thebpanya P. Long-term exclosure of large terrestrial vertebrates: implications of defaunation for seedling demographics in the Amazon rainforest. Biological Conservation 2013; 163: 115–121. [Google Scholar]
- 8.Levi T, Peres CA. Dispersal vaccum in the seedling recruitment of a primate-dispersed Amazonian tree. Biological Conservation 2013; 163: 99–106. [Google Scholar]
- 9.Peres CA, Dolman PM. Density compensation in neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 2000; 122: 175–189. [DOI] [PubMed] [Google Scholar]
- 10.Valiente-Banuet A, Aizen MA, Alcántara JM, Arroyo J, Cocucci A, Galetti M, et al. Beyond species loss: the extinction of ecological interactions in a changing world. Functionnal Ecology 2015; 29: 299–307. [Google Scholar]
- 11.Lewis SL, Edwards DP, Galbraith D. Increasing human dominance of tropical forests. Science 2015; 349: 827–832. 10.1126/science.aaa9932 [DOI] [PubMed] [Google Scholar]
- 12.Eva HD, Achard F, Beuchle R, de Miranda E, Carboni S, Seliger R, et al. Forest cover changes in tropical south and central America from 1990 to 2005 and related carbon emissions and removals. Remote Sensing 2012; 4: 1369–1391. [Google Scholar]
- 13.Goodwin A, editors. Principles of Precambrian Geology. Academic Press, New York, 1996; 319 pp. [Google Scholar]
- 14.Hammond DS. Ancient land in a modern world In: Hammond D.S. (ed). Tropical Forests of the Guiana Shield CABI Publishing, Cambridge, 2005, pp. 1–14. [Google Scholar]
- 15.Taber A, Chalukian SC, Altrichter M, Minkowski K, Lizárraga L, Sanderson E, et al. El Destino de los Arquitectos de los Bosques Neotropicales: Evaluación de la Distribución y el Estado de Conservación de los Pecaríes Labiados y los Tapires de Tierras Bajas. 2008; New York, Pigs, Peccaries and Hippos Specialist Group (IUCN/SSC); Tapir Specalist Group (IUCN/SSC); Wildlife Conservation Society; and Wildlife Trust. [Google Scholar]
- 16.Groenendijk J. A review of the distribution and conservation status of the giant otter (Pteronura brasiliensis), with special emphasis on the Guayana Shield Region Netherlands Commitee for IUCN, 1998. Amsterdam. [Google Scholar]
- 17.Rheingantz ML, Saraiva de Menezes JF, de Thoisy B. Defining Neotropical otter Lontra longicaudis distribution, conservation priorities and ecological frontiers. Tropical Conservation Science 2014; 7: 214–229. [Google Scholar]
- 18.Zeller KA. Jaguars in the New Millennium Data Set Update: The State of the Jaguar in 2006. Wildlife Conservation Society, Bronx, New York; 2007. [Google Scholar]
- 19.Hammond DS, Gond V, de Thoisy B, Forget PM, DeDijn B. Causes and consequences of a tropical forest gold rush in the Guiana Shield, South America. Ambio 2007; 36: 661–670. [DOI] [PubMed] [Google Scholar]
- 20.Leisher C, Touval J, Hess SM, Boucher TM, Reymoudin L. Land and forest degradation inside protected areas in Latin America. Diversity 2013; 5: 779–795. [Google Scholar]
- 21.Alvarez-Berrios NL, Aide MT. Global demand for gold is another threat for tropical forests. Environmental Research Letter 2015; 10: 014006. [Google Scholar]
- 22.de Thoisy B, Renoux F, Julliot C. Hunting in northern French Guiana and its impacts on primates communities. Oryx 2005; 39: 149–157. [Google Scholar]
- 23.de Thoisy B, Richard-Hansen C, Peres CA. Impacts of subsistence game hunting on amazonian primates In Garber Paul A., Estrada Alejandro, Bicca-Marques Júlio César, Heymann Eckhard W. and Strier Karen B. (eds). South American Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation. 2009; Book Series Developments in Primatology: Progress and Prospects, Springer Press, Chap 15, pp 389–412. [Google Scholar]
- 24.de Thoisy B, Richard-Hansen C, Goguillon B, Joubert P, Obstancias J, Winterton P, et al. Rapid evaluation of threats to biodiversity: human footprint score and large vertebrate species responses in French Guiana. Biodiversity and Conservation 2010; 19: 1567–1584. [Google Scholar]
- 25.Torres NM, De Marco P Jr, Santos T, Silveira L, Jacomo ATA, Diniz-Filho JAF. Can species distribution modelling provide estimates of population densities? A case study with the jaguar in the Neotropics. Diversity and Distributions 2012; 18: 615–627. [Google Scholar]
- 26.Farhadinia MS, Ahmadi M, Sharbafi E, Khosravi S, Alinezhad H, MacDonald DW. Leveraging trans-boundary conservation partnerships: persistence of Persian leopard (Panthera pardus saxicolor) in the Iranian Caucasus. Biological Conservation 2015; 191: 770–778. [Google Scholar]
- 27.Caso A, Lopez-Gonzalez C, Payan E, Eizirik E, de Oliveira T, Leite-Pitman R, et al. 2008. Panthera onca. The IUCN Red List of Threatened Species. 2008; Version 2014.3. <www.iucnredlist.org>. Downloaded on 11 March 2015.
- 28.Estes JA, Terborgh J, Brashares J, Power ME, Berger J, Bond WJ, et al. Trophic downgrading of planet earth. Science 2011; 333: 331–306. [DOI] [PubMed] [Google Scholar]
- 29.de Barros AE, MacDonald EA, Matsumoto MH, Paula RCP, Nijhawan S, Malhi Y, et al. Identification of areas in Brazil that optimize conservation of forest carbon, jaguars, and biodiversity. Conservation Biology 2014; 28: 580–593. 10.1111/cobi.12202 [DOI] [PubMed] [Google Scholar]
- 30.Tobler MW, Powell CVN. Estimating jaguar densities with camera-traps: problems with current designs and recommendations for future studies? Biological Conservation 2013; 159: 109–118. [Google Scholar]
- 31.de Thoisy B, Brosse S, Dubois MA. Assessment of large-vertebrate species richness and relative abundance in Neotropical forest using line-transect censuses: what is the minimal effort required? Biodiversity and Conservation 2008; 17: 2627–2644. [Google Scholar]
- 32.Gond V, Brognoli C. Télédétection et aménagement du territoire: localisation et identification des sites d’orpaillage en Guyane française. Bois et Forêts des Tropiques 2005; 286: 5–13. [Google Scholar]
- 33.Briant G, Gond V, Laurance S. Habitat fragmentation and the desiccation of forest canopies: A case study from eastern Amazonia. Biological Conservation 2010; 143: 2763–2769. [Google Scholar]
- 34.Bourbier L, Cornu G, Pennec A, Brognoli C, Gond V. Large scale forest opening estimation using remote sensing in Central Africa. Bois et Forêts des Tropiques 2013; 315: 3–9. [Google Scholar]
- 35.Pithon S, Jubelin G, Guitet S, Gond V. Statistical based method for logging-related canopy gap detection using high resolution optical remote sensing. International Journal of Remote Sensing 2013; 34: 700–711. [Google Scholar]
- 36.De Sy V, Herold M, Achard F, Beuchle R, Clevers JGPW, Lindquist E, et al. Land use patterns and related carbon losses following deforestation in South America. Environmental Research Letters 2015; 10: 124004. [Google Scholar]
- 37.Rose CA, Byler D, Eastman JR, Fleishman E, Geller G, Goetz S, et al. Ten ways remote sensing can contribute to conservation. Conservation Biology 2015; 29: 350–359. 10.1111/cobi.12397 [DOI] [PubMed] [Google Scholar]
- 38.Silver S, Ostro L, Marsh L, Maffei L, Noss A, Kelly M. The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx 2004; 38: 148–154. [Google Scholar]
- 39.Lindeman JC, Mori SA. The Guianas Pp. 375–390 in Campbell DG, Hammond HD, editors. Floristic Inventories of Tropical Countries: The Status of Plants Systematics, Collections and Vegatation, Plus Recommendations for the Future. 1989; New York: New York Botanical Garden. [Google Scholar]
- 40.Guitet S, Freycon V, Brunaux O, Pélissier R, Sabatier D, Couteron P. Geomorphic control of rain-forest floristic composition in French Guiana: more than a soil filtering effect? Journal of Tropical Ecology 2016; 32: 22–40. [Google Scholar]
- 41.Gond V, Freycon V, Molino JF, Brunaux O, Ingrassia F, Joubert P, et al. Broad-scale spatial pattern of forest landscapes types in the Guiana shield. International Journal of Applied Earth Observation and Geoinformation 2011; 13: 357–367. [Google Scholar]
- 42.Guitet S, Pélissier R, Brunaux O, Jaouen G, Sabatier D. Geomorphological landscape features explain floristic patterns in French Guiana rainforest. Biodiversity and Conservation 2015; 24:2015–2037. [Google Scholar]
- 43.de Granville JJ. Phytogeographical characteristics of the Guianan forests. Taxon 19888; 37: 578–594. [Google Scholar]
- 44.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling 2006; 190: 231–259. [Google Scholar]
- 45.Wisz MS, Hijmans RJ, Li J, Peterson AT, Guisan A. Effects of sample size on the performance of species distribution models. Diversity and Distributions 2008; 14: 763–773. [Google Scholar]
- 46.Dudík M, Schapire RE, Phillips SJ. Correcting sample selection bias in maximum entropy density estimation. Advances in Neural Information Processing Systems 2005; 18: 323–330. [Google Scholar]
- 47.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 2005; 25: 1965–1978. [Google Scholar]
- 48.Farr TG, Rosen PA, Caro E, Crippen R, Duren R, Hensley S, et al. The shuttle radar topography mission. Reviews of Geophysics 2007; 45: Rg2004 [Google Scholar]
- 49.Guitet S, Cornu JF, Brunaux O, Betbeder J, Carozza JM, Richard-Hansen C. Landform and landscape mapping, French Guiana (South America). Journal of Maps 2013; 9: 325–335. [Google Scholar]
- 50.Montoya D, Purves DW, Urbieta IR, Zavala MA. Do species distribution models explain spatial structure within tree species ranges? Global Ecology and Biogeography 2009; 18:662–673. [Google Scholar]
- 51.Bradley AP. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognition Letter 1997; 30:1145–1159. [Google Scholar]
- 52.Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography 2008; 17:145–151. [Google Scholar]
- 53.Raes N, Steege H. A null-model for significance testing of presence-only species distribution models. Ecography 2007; 30:727–736. [Google Scholar]
- 54.Guitet S, Pithon S, Brunaux O, Jubelin G, Gond V. Impacts of logging on the canopy and the consequences for forest management in French Guiana. Forest Ecology and Management 2012; 277: 124–131. [Google Scholar]
- 55.White GC, Anderson DR, Burnham KP, Otis DL. Capture-recapture and removal methods for sampling closed populations. Los Alamos National Laboratory Publications; 1982; LA-8787-NERP. 235pp. [Google Scholar]
- 56.Avezedo FCC, Murray DL. Spatial organization and food habits of jaguars (Panthera onca) in a floodplain forest. Biological Conservation 2007; 137: 391–402. [Google Scholar]
- 57.Foster RJ, Harmsen BJ, Valdes B, Pomilla C, Duncaster CP. Food habits of sympatric jaguars and pumas across a gradient of human disturbance. Journal of Zoology 2010; 280: 309–318. [Google Scholar]
- 58.Richard-Hansen C, Jaouen G, Denis T, Brunaux O, Marcon E, Guitet S. Landscape patterns influence communities of medium-to large-bodied vertebrates in undisturbed terra firme forests of French Guiana. Journal of Tropical Ecology 2015; 31:423–436. [Google Scholar]
- 59.Richard-Hansen C, Vié JC, Vidal N, Kéravec J. Body measurements on 40 species of mammals from French Guiana. Journal of Zoology 1999; 247:419–428. [Google Scholar]
- 60.Lefsky MA, Harding DJ, Keller M, Cohen WB, Carabajal CC, Espirito-Santo FDB, et al. Estimates of forest canopy height and aboveground biomass using ICESat. Geophysical Research Letters 2005; 32: L22S02 [Google Scholar]
- 61.Harding DJ, Carabajal CC. ICESat waveform measurements of within-footprint topographic relief and vegetation vertical structure. Geophysical Research Letters 2005; 32: L21S10 [Google Scholar]
- 62.Chen Q. Retrieving vegetation height of forests and woodlands over mountainous areas in the Pacific Coast region using satellite laser altimetry. Remote Sensing of Environment 2010; 114: 1610–1627. [Google Scholar]
- 63.Hilbert C, Schmullius C. Influence of surface topography on ICESat/GLAS forest height estimation and waveform shape. Remote Sensing 2012; 4: 2210–2235. [Google Scholar]
- 64.Fayad I, Baghdadi N, Bailly JS, Barbier N, Gond V, El Hajj M, et al. Canopy height estimation in French Guiana using LiDAR ICESat/GLAS data using principal component analysis and random forest regressions. Remote Sensing 2014; 6: 11883–11914. [Google Scholar]
- 65.Guitet S, Hérault B, Molto Q, Brunaux O, Couteron P. Spatial structure of above-ground biomass limits accuracy of carbon mapping in rainforest but large scale forest inventories can help to overcome. PloS ONE 2015; 10: e0138456 10.1371/journal.pone.0138456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breiman L. Random forests. Machine Learning 2001; 45: 5–32. [Google Scholar]
- 67.Delor C, Lahondère D, Egal E, Marteau P. Carte géologique de la France à 1: 500 000 2001; Département de la Guyane, Guyane, BRGM, Orléans. [Google Scholar]
- 68.Sexton JO, Noojipady P, Song XP, Feng M, Song DX, Kim DH, et al. Conservation policy and the measurement of forests. Nature Climate Change 2015; 10.1038/NCLIMATE2816 [DOI] [Google Scholar]
- 69.Noss AJ, Kelly MJ, Camblos HB, Rumiz DI. Pumas y jaguares simpátricos: datos de trampas-cámara en Bolivia y Belize MEMORIAS: Manejo de Fauna silvestre en Amazonia y Latinoamérica 2006; 229–237. [Google Scholar]
- 70.Conde DA, Colchero F, Zarza H, Christensen NL Jr, Sexton JO, Manterola C, et al. Sex matters: modeling male and female habitat differences for jaguar conservation. Biological Conservation 2010; 143: 1980–1988. [Google Scholar]
- 71.Gonzalez CA, Miller BJ. Do jaguars (Panthera onca) depend on large prey? Western North American Naturalist 2002; 62: 218–222. [Google Scholar]
- 72.Lopes MA, Ferrari SF. Effects of human colonization on the abundance and diversity of mammals in Eastern Brazilian Amazonia. Conservation Biology 2000; 14: 1658–1665 [DOI] [PubMed] [Google Scholar]
- 73.Wright SJ, Zeballos H, Dominguez I, Gallardo MM, Moreno MC, Ibanez R. Poachers alter mammal abundance, seed dispersal and seed predation in a neotropical forest. Conservation Biology 2000; 14: 227–239. [Google Scholar]
- 74.Haugassen T, Peres CA. Mammal assemblage structure in Amazonian flooded and unflooded forests. Journal of Tropical Ecology 2005; 21: 133–145. [DOI] [PubMed] [Google Scholar]
- 75.Haugassen T, Peres CA. Population abundance and biomass of large bodied birds in Amazonian flooded and unflooded forests. Bird Conservation International 2008; 18: 87–101. [Google Scholar]
- 76.Clément L, Catzeflis F, Richard-Hansen C, Barrioz S, de Thoisy B. Conservation interests of spatial distribution modelling applied to large vagile neotropical mammals. Tropical Conservation Science 2014; 7: 193–213. [Google Scholar]
- 77.Kelly MJ, Rowe C. Analysis of 5 years data from Rio Bravo Conservation and Management Area (RBCMA) and one year of data from Gallon Jug/Yalbac Ranch on trap rates for predator and prey, including jaguar density estimates in unlogged versus sustainably logged areas. Report for Rio Bravo Conservation and Management Area. Department of Fish and Wildlife Conservation, 2014; Virginia Tech. Blacksburg, VA. USA.
- 78.Tobler MW, Hibert F, Debeir L, Richard-Hansen C. Estimates of density and sustainable harvest of the lowland tapir Tapirus terrestris in the Amazon of French Guiana using a Bayesian spatially explicit capture–recapture model. Oryx 2014; 48: 410–419. [Google Scholar]
- 79.Thornton D, Zeller K, Rondinini C, Boitani L, Crooks K, Burdett C, et al. Assessing the umbrella value of a range-wide conservation network for jaguars (Panthera onca). Ecological Application 2016; 26: 1112–1124. [DOI] [PubMed] [Google Scholar]
- 80.Pitman RT, Swanepoel LH, Hunter L, Slotow R, Balme GA. The importance of refugia, ecological traps and scale for large carnivore management. Biodiversity and Conservation 2015; 24: 1975–1987. [Google Scholar]
- 81.Boron V, Tzanopoulos J, Gallo J, Barragan J, Jaimes-Rodriguez L, Schaller G, et al. Jaguar Densities across Human-Dominated Landscapes in Colombia: The Contribution of Unprotected Areas to Long Term Conservation. PloS One 2016; 11 e0153973 10.1371/journal.pone.0153973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schipper J, Clarke G, Allnutt T. Conservation Planning in a Tropical Wilderness: Opportunities and Threats in the Guianan Ecoregion Complex. 2007; USDA Forest Service Proceedings RMRS-P-49.
- 83.Rabinowitz A, Zeller KA. A range-wide model of landscape connectivity and conservation for the jaguar, Panthera onca. Biological Conservation 2010; 143: 939–945. [Google Scholar]
- 84.Gilpin ME. Population viability analysis. Endangered Species Update 1989; 6–10: 15–18. [Google Scholar]
- 85.Picard N, Gourlet-Fleury S, Forni É. Estimating damage from selective logging and implications for tropical forest management. Canadian Journal of Forest Research, 2012; 42: 605–613. [Google Scholar]
- 86.Peres CA. Effects of Subsistence hunting on vertebrate community structure in Amazonian Forests. Conservation Biology 2000; 14: 240–253. [Google Scholar]
- 87.Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, et al. Trophic downgrading of planet earth. Science 2011; 333: 331–306. [DOI] [PubMed] [Google Scholar]
- 88.Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, et al. Status and ecological effects of the world’s largest carnivores. Science 2014; 343: 1241484 10.1126/science.1241484 [DOI] [PubMed] [Google Scholar]
- 89.Hayward MW, Kamler F, Montgomery RA, Newlove A, Rostro-García S, Sales LP, et al. Prey preferences of the Jaguar Panthera onca reflect the Post-Pleistocene demise of large prey. Frontiers in Ecology and Evolution 2016; 3: 148. [Google Scholar]
- 90.Garmendia A, Arroyo-Rodríguez V, Estrada A, Naranjo EJ, Stoner KE. Landscape and patch attributes impacting medium-and large-sized terrestrial mammals in a fragmented rain forest. Journal of Tropical Ecology 2013;, 29:331–344. [Google Scholar]
- 91.Caro T, Engili A, Fitzeherbert E, Gardner T. Preliminary assessment of the flagship species concept at a small scale. Animal Conservation 2004; 7: 63–70. [Google Scholar]
- 92.Hudson LN, Newbold T, Contu S, Hill SLL, Lysenko I, De Palma A, et al. The PREDICTS database: a global database of how local terrestrial biodiversity responds to human impacts. Ecology and Evolution 2014; 4: 4701–4735. 10.1002/ece3.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turner W, Rondinini C, Pettorelli N, Mora B, Leidner AK, Szantoi Z, et al. Free and open-access satellite data are key to biodiversity conservation. Biological Conservation 2015; 182: 173–176. [Google Scholar]
- 94.Toogood M. Engaging publics: biodiversity data collection and the geographies of citizen science. Geography Compass 2013; 7/9: 611–621. [Google Scholar]
- 95.Couvet D, Prévot AC. Citizen-science programs: towards transformative biodiversity governance. Environmental Development 2015; 13: 39–45 [Google Scholar]
- 96.Loos J, Horcea-Milcua AI, Kirkland P, Hartel T, Osváth-Ferencz M, Fischer J. Challenges for biodiversity monitoring using citizen science in transitioning social–ecological systems. Journal for Nature Conservation 2015; 26: 45–48 [Google Scholar]
- 97.Zimmerman NE, Edwards TC Jr, Graham CH, Pearman PB, Svenning JC. New trends in Species Distribution Models. Ecography 2010; 33: 985–989. [Google Scholar]
- 98.Moreira-Arce D, Vergara PM, Boutin S, Carrasco G, Briones R, Soto GE, et al. Mesocarnivores respond to fine-grain habitat structure in a mosaic landscape comprised by commercial forest plantations in southern Chile. Forest Ecology and Management 2016; 369: 135–143. [Google Scholar]
- 99.Vierling KT, Vierling LA, Gould WA, Martinuzzi S, Clawges RM. Lidar: shedding new light on habitat characterization and modeling. Frontiers in Ecology and the Environment 2008; 6: 90–98. [Google Scholar]
- 100.Nagendra H, Lucas R, Honrado JP, Jongman RH, Tarantino C, Adamo M, et al. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecological Indicators 2013; 33: 45–59. [Google Scholar]
- 101.Kehl TN, Todt V, Veronez MR, Cazella SC. Amazonian forest deforestation detection tool in real time using Artificial Neural Networks and Satellite Images. Sustainability 2012; 4: 2566–2573. [Google Scholar]
- 102.Barbier N, Couteron P, Proisy C, Malhi Y. The variation of apparent crown size and canopy heterogeneity across lowland Amazonian forests. Global Ecology and Biogeography 2010; 19: 72–84. [Google Scholar]
- 103.Hammond DS, Gond V, Baider C, Florens F, Persand S, Laurance S. Threats to environmentally sensitive areas from peri-urban expansion in Mauritius. Environmental Conservation 2015; 10.1017/S0376892914000411 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Data are from the current study and are provided as Supporting Information.