Abstract
This article provides a definitive review of experimental studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus (CCHFV), the etiologic agent of Crimean-Congo hemorrhagic fever (CCHF), beginning with the first recognized outbreak of the human disease in Crimea in 1944. Published reports by researchers in the former Soviet Union, Bulgaria, South Africa, and other countries where CCHF has been observed show that CCHFV is maintained in nature in a tick-vertebrate-tick enzootic cycle. Human disease most commonly results from the bite of an infected tick, but may also follow crushing of infected ticks or exposure to the blood and tissues of infected animals during slaughter. Wild and domestic animals are susceptible to infection with CCHFV, but do not develop clinical illness. Vertebrates are important in CCHF epidemiology, as they provide blood meals to support tick populations, transport ticks across wide geographic areas, and transmit CCHFV to ticks and humans during the period of viremia. Many aspects of vertebrate involvement in the maintenance and spread of CCHFV are still poorly understood. Experimental investigations in wild animals and livestock provide important data to aid our understanding of CCHFV ecology. This article is the second in a series of reviews of more than 70 years of research on CCHF, summarizing important findings, identifying gaps in knowledge, and suggesting directions for future research.
Keywords: Crimean-Congo hemorrhagic fever, bunyavirus, tick-borne, viral hemorrhagic fever, transmission
1. Scope of this review
This article reviews experimental studies of the roles of wild and domestic vertebrates in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus (CCHFV) that have been published since the first recognized outbreak of the disease in 1944. It is the second article in a series that aims to provide a definitive account of research on Crimean-Congo hemorrhagic fever (CCHF), which has not been thoroughly examined since the monumental monograph by the noted acarologist Harry Hoogstraal was published in 1979 (1). The first paper reviewed seroepidemiologic studies of the geographic distribution, host range, and frequency of CCHFV infection of wild and domestic animals (2). Additional articles in this series will review the role of ticks in the maintenance of CCHFV and its transmission to vertebrates including humans, and the attempts to replicate important features of CCHF through experimental infection of laboratory animals. Readers are also referred to a general review of the epidemiology, viral genetic diversity, and other features of CCHF; a review of the public health implications of CCHF; and a recent review of experimental molecular studies of the virus (3–5).
2. Background: maintenance and transmission of CCHFV
CCHF was first recognized in the Crimean peninsula in 1944–1945, when more than 200 cases of a severe febrile illness occurred in soldiers working to restore agricultural production in farmland abandoned during the German occupation (6). Mikhail Chumakov, who had previously characterized the maintenance cycle of tick-borne encephalitis in the Soviet Far East, led the team of scientists sent to investigate the outbreak. An association of what was then called Crimean disease with tick bites was quickly recognized, and a viral etiology was established through experimental infection studies via inoculation of filtered tick extracts (7,8).
The Crimean outbreak exemplified the major features of CCHF. The disease occurred exclusively in the agricultural steppe, while no cases were reported in towns or wooded areas. The outbreak coincided with a period of unusually high tick numbers in the spring and early summer of 1944, and most patients reported a tick bite prior to illness. Although a large number of soldiers became ill, there was no evidence of person-to-person transmission. As described in the 1945 expedition report (6), more than 3000 blood-sucking arthropods were collected, but the most common parasite was the tick Hyalomma marginatum, which is now recognized to be the principal vector of CCHFV in Russia and adjacent areas.
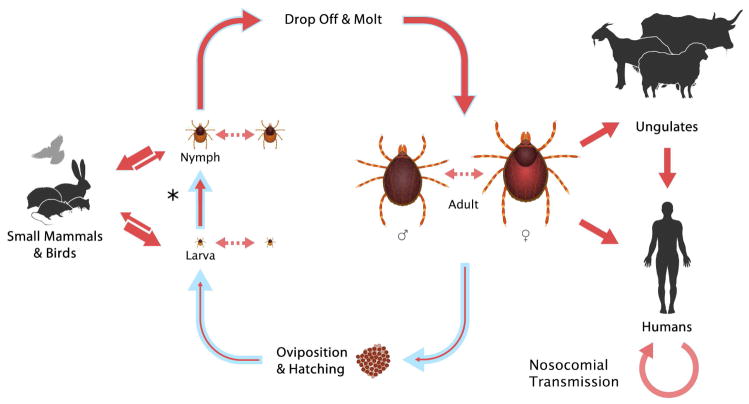
CCHFV (family Bunyaviridae; genus Nairovirus) circulates silently in a tick-vertebrate-tick enzootic cycle (1,3,9) (Figure 1), with a brief viremia in vertebrates and prolonged viral maintenance in ticks by transstadial and transovarial transmission, and, less efficiently, by venereal transmission (9). Nairoviruses are a genetically highly diverse group, and congruence between phylogenies supports coevolution of nairoviruses and ticks (10,11). Tick life cycles are dependent on animal hosts, and are defined by the number of hosts they feed on while developing through the three parasitic life stages (larva, nymph, adult) before the adult female drops off the host for oviposition after the final blood meal (Figure 2). Over 30 tick species, including one-, two-, and three-host species, have been implicated in CCHFV ecology (1,12); CCHFV vector and reservoir competence remains unknown for many of these species. One-host ticks (e.g., Rhipicephalus annulatus, Rhipicephalus decoloratus, Rhipicephalus microplus) remain on one host, typically a large mammal like a cow, during all life stages. Two-host ticks (e.g., H. marginatum complex of species, Hyalomma detritum, Hyalomma anatolicum) feed and remain on the first host, typically a small mammal, during the larval and nymphal life stages, and then drop off and attach to a different host, usually a larger mammal, as adults for a final blood meal. Three-host ticks (e.g., Ixodes ricinus, Haemaphysalis punctata, Dermacentor marginatus) drop off and reattach to a new host during each life stage, generally using small vertebrates for the two initial stages and large mammals for the final meal. Consideration of tick and vertebrate host ecology in CCHFV is complex. While some vector species may locally exhibit a narrow host range (e.g., Hyalomma dromedarii for dromedary camels, and Hyalomma aegyptium for Testudo species tortoises [13]), most have a wide host range. For example, I. ricinus has over 300 putative hosts, including many birds and lizards (14–16).
Figure 1.
Life cycle of Hyalomma spp. ticks and vertical and horizontal transmission of Crimean-Congo hemorrhagic fever virus (CCHFV) (3). The course of the tick life cycle is indicated with blue arrows. Upon hatching, larvae find a small animal host for their first blood meal (hematophagy). Depending on the tick species, the larvae either remain attached to their host following engorgement and molt in place (two-host ticks) or drop off to molt (three-host ticks); this transition is marked by an asterisk. The nymphs then either continue to feed on the animal on which they molted (two-host ticks) or attach to a new small vertebrate host (three-host ticks). Upon engorgement, nymphs of all species drop off their host and molt into adults. Adult ticks then find a large animal for hematophagy, and mate while attached to the host. After taking a blood meal, the engorged females drop off and find a suitable location for ovipositing. During the tick life cycle, CCHFV can be transmitted between ticks and mammals (solid red arrows), and directly between ticks through co-feeding (dashed arrows). For each kind of virus transfer, the thickness of the arrow indicates the efficiency of transmission. Humans can become infected through the bite of an infected tick or through exposure to the body fluids of a viremic animal or an infected person.
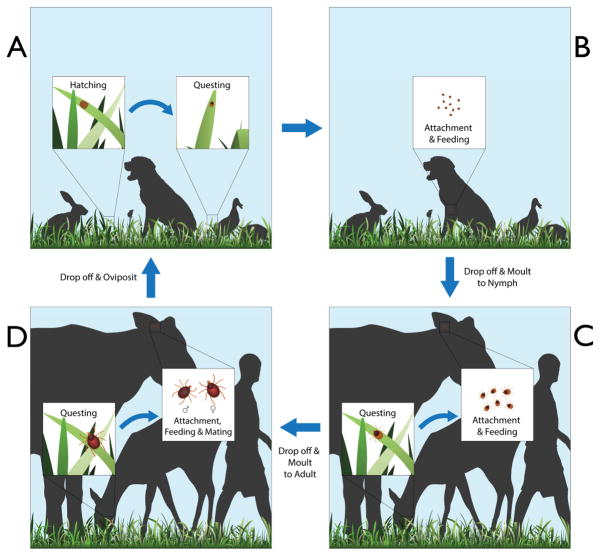
Figure 2.
Idealized life cycle of an Ixodes spp. tick with three hosts, showing the three stages (larva, nymph, and adult) (16). The eggs incubate in ground litter (A), protected against stressful environmental conditions (e.g., water losses). When the larvae hatch, they climb the vegetation, questing for a host (A→B). Larvae feed on such hosts (B), and when engorged, drop off to the ground and molt to nymphs (B→C). The nymphs quest for a second host, feed, and molt again off the host (C→D). The resulting adults attach to a third host, feed, and mate, and the females drop off for ovipositing on the ground.
Because wild and domestic animals infected with CCHFV do not show signs of illness, serological surveys have been the principal source of information to identify species exposed to the virus and to monitor areas with natural virus transmission. However, tests performed on serum collected from vertebrates do not provide data on characteristics and kinetics of infection. Experimental infection studies have been performed in a variety of wild and domestic animals to assess susceptibility to infection, duration of viremia, antibody response, absence or presence of clinical disease, and contribution of vertebrate species as hosts in CCHFV maintenance and transmission. Data from these studies are useful in assessing the risk of CCHF emergence in new geographic areas, focusing One Health countermeasures for reducing endemic viral transmission (e.g., by targeted acaricide treatment), assessing risk in cases of direct human exposure to potentially infected animal tissues and body fluids, identifying gaps in knowledge, and directing future research efforts (4,17–20).
Notably, these clinical infection studies and almost all of the important work on CCHFV were performed after the application of the pivotal newborn white mouse (NWM) isolation technique (21,22). Attempts to recover the etiologic agent were largely unsuccessful for more than two decades after the Crimean outbreak. In efforts to isolate virus from animals prior to use of the NWM technique, clinical disease was often attributed to the presence of a contaminating organism (e.g., studies in cats [6,25]). In 1964–1965, CCHFV isolated from the blood of patients was successfully passaged in tissue culture; passage was confirmed by using the fluorescent antibody method and interference phenomenon using vesicular stomatitis virus and Chikungunya virus (24,25). However, in the late 1950s and 1960s, arbovirus researchers discovered that many viruses are more successfully propagated through intracranial infection of NWM (21,22). The introduction of this simple but highly sensitive method revolutionized CCHFV research by providing a means to isolate and quantitate virus recovered from human patients, animals, and ticks, and facilitating the development of serologic tests and other research tools (1,26–29).
3. Experimental infection in wild mammals
Wild mammals are important hosts for ticks and likely aid in CCHFV amplification and transmission from infected to naïve co-feeding ticks. In general, CCHFV replicates and produces a short viremic period in small mammals (2–15 days), followed by development of anti-CCHFV antibodies (Table 1). Thus, small mammals do not appear to serve efficiently as long-term CCHFV reservoirs. They do, however, play an important role in CCHFV ecology, and population increases (e.g., in hares) have been associated with disease outbreaks. Of note, the studies detailed below are limited to small mammalian species, and although serological data do exist (as detailed in Spengler et al. [2]), to our knowledge, no experimental studies have been performed in larger wild mammalian species.
Table 1. CCHFV experimental infection in small mammals.
None of the studies reported clinical signs in inoculated animals. LD50 were determined by intracranial inoculation of newborn white mice (NWM).
| Common name | Taxonomic name (class/order) | Virus strain | Route/Dose | Viremia | Antibody response | Year | Ref. |
|---|---|---|---|---|---|---|---|
| European hedgehog | Erinaceus europaeus (Mammalia/Erinaceomorpha) | Sudarkina | SC, 1 × 107–1 × 108 LD50 | No viremia detected (5–13 dpi) | No (CF or AGPD, up to 1 month) | 1975 | (38) |
| Long-eared hedgehog | Hemiechinus auritus (Mammalia/Erinaceomorpha) | Sudarkina | SC, 1 × 107–1 × 108 LD50 | Viremia, 2–6 dpi (max 4–6 dpi) | No (CF or AGPD, up to 1 month) | 1975 | (38) |
| Long-eared hedgehog | Hemiechinus auritus (Mammalia/Erinaceomorpha) | NR | IV/IM | Viremia (details NR) | NR | 1971 | (34) |
| South African hedgehog | Atelerix frontalis (Mammalia/Erinaceomorpha) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | No viremia detected | Yes | 1989 | (36) |
| European hare | Lepus europaeus (Mammalia/Lagomorpha) | Sudarkina | Intracardiac, SC, or ID (n = 2, each), 1 × 105 LD50 | Intracardiac inoculation: viremia detected at 2, 4, 5, and 9 dpi (but not 6, 7, 8 dpi); SC inoculation: viremia at 1, 2, and 5 dpi; ID inoculation: at 1–10 dpi | Yes, CF antibody at 7 dpi; high CF Ab titers detectable 28–45 dpi (study period) | 1972 | (35) |
| European hare | Lepus europaeus (Mammalia/Lagomorpha) | NR | IV/IM, 1 × 1010 LD50 | Viremia up to 15 dpi (max of (max of 2 × 105 LD50/mL at 4 dpi) | NR | 1971 | (34) |
| Hare | Lepus sp. (Mammalia/Lagomorpha) | NR | IV/IM | Viremia (7.9 × 104–2.0 × 105 LD50/mL at 4–6 dpi) | NR | 1975 | (79) |
| Scrub hare | Lepus saxatilis (Mammalia/Lagomorpha) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | Viremia, 1–7 dpi (max 1.6 × 104 LD50/mL) | Yes | 1989 | (36) |
| Bushveld gerbil | Gerbilliscus leucogaster (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | Viremia, 1–7 dpi (max 5 × 102 LD50/mL) | Yes | 1989 | (36) |
| Ground squirrel (little suslik) | Spermophilus pygmaeus (Mammalia/Rodentia) | Sudarkina, Hp-15 Zhr | IC, 4 × 104–1.3 × 105 LD50; or IM/SC, 5 × 105–1.6 × 106 LD50 | Virus isolated from blood and organs of all animals 2–7 dpi (but not later) | NR | 1972 | (37) |
| Cape ground squirrel | Xerus inauris (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | Viremia 2–8 dpi (max 5 × 102 LD50/mL) | Yes | 1989 | (36) |
| Highveld gerbil | Gerbilliscus brantsii (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | No viremia | Yes | 1989 | (36) |
| Southern multimammate mouse | Mastomys coucha (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | No viremia | Yes | 1989 | (36) |
| Multimammate mouse | Mastomys natalensis (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | No viremia | Yes | 1989 | (36) |
| Namaqua gerbil | Desmodillus auricularis (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | NR | Yes | 1989 | (36) |
| Red veld rat | Aethomys chrysophilus (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | Viremia, 1–3 dpi (max 1 × 104 LD50/mL) | Yes | 1989 | (36) |
| Four-striped grass mouse | Rhabdomys pumilio (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | Viremia, 1 day duration (5 dpi), 3.2 × 101 LD50/mL | No* | 1989 | (36) |
| White-tailed rat | Mystromys albicaudatus (Mammalia/Rodentia) | SPU 4/81 | SC, 1 × 106–2.5 × 106 LD50 | Viremia, 1–6 dpi (max 5.0 × 103 LD50/mL) | Yes | 1989 | (36) |
Abbreviations: AGPD, agar gel diffusion precipitation; CF, complement fixation; dpi, days post infection; IC, intracranial; ID, intradermal; IM, intramuscular; IP, intraperitoneal; IV, intravenous; NR, not reported; SC, subcutaneous.
CCHFV antibodies were not detected in animals euthanized up to 8 dpi.
3.1. Hares
Small mammals provide blood meals for two- and three-host ticks like D. marginatus and Hyalomma asiaticum. The initial report from the Crimean outbreak implicated the European hare (Lepus europaeus) as central in maintaining CCHFV; the L. europaeus population reportedly exploded during the original CCHF outbreak in the Crimea (1). Indeed, hares are widely distributed and are still considered to be primary hosts of several CCHFV vectors in many CCHF-endemic areas in Europe. Moreover, of the 11 reported isolates of CCHFV from animals, three were from L. europaeus in the Crimea region of Ukraine (30). An overgrowth of vegetation due to cessation of farming, and an expansion of the hare population (and the associated abundance of ticks) were described during several CCHF outbreaks, including the initial Crimean outbreak and outbreaks in the former Soviet Union, Bulgaria, Kosovo, and Turkey (31–33).
3.1.1. Former Soviet Union
Hares were included in early experimental investigations of CCHFV persistence in the tick. Zgurskaya et al. (1971) used hares inoculated intravenously (IV) and intramuscularly (IM) as infection sources for Hyalomma ticks (34). NWM were used to assess viremia in the hares 4–18 days post infection (dpi). Viremia was detected in L. europaeus until 15 dpi, with peak viremia of 1.2 × 105 LD50/mL at 4 dpi. (LD50 in this and subsequently described studies refers to the virus dose lethal to 50% of inoculated NWM.) In additional studies on threshold CCHFV levels in Hyalomma ticks by Zgurskaya (1975), a hare (species not reported; n = 1) was inoculated IV and IM with a NWM brain suspension. Viremia was confirmed at 4–6 dpi (transmission studies further detailed in Section 6.2).
Perelatov et al. (1972) studied hares trapped on a hunting farm in the Ust’-Donets region (Russia, Rostov Region). Six 1–2-year-old naïve European hares were inoculated with 105 LD50 of strain Sudarkina CCHFV (35). Infection was performed via intracardiac, subcutaneous (SC), or intradermal (ID) inoculation, with two animals infected by each inoculation route. After infection, no clinical signs were noted during the 2-year observation period. Viremia was investigated in whole or diluted blood. Detection and magnitude of viremia varied by route of inoculation: with SC inoculation, viremia was lowest and was detected at 1, 2, and 5 dpi; intracardiac inoculation produced intermediate viremia levels detectable 2, 4, 5, and 9 dpi, but not 6, 7, and 8 dpi; and ID inoculation resulted in the highest viremia levels detectable 1–10 dpi. Why viremia was detected intermittently is not clear, but is likely explained by the sensitivity of assays used in those experiments. Antibodies were detected by complement fixation (CF) beginning 7 dpi and throughout an extended observation period (28–45 dpi); data were not detailed for each inoculation group (35).
3.1.2. Africa
In agreement with the above studies, Shepherd et al. (1989) reported a comparable viremia in scrub hares (Lepus saxatilis) inoculated SC with CCHFV strain SPU 4/81. Viremia was detected 1–7 dpi, with highest virus titers (1.6 × 104 LD50/mL) detected between 2–5 dpi (36). Along with vector studies and knowledge of host factors like foraging behavior and geographic distribution, these limited experimental infection investigations support the hypothesis that hares can replicate CCHFV, and provide antibody data for interpreting serological studies.
3.2. Ground squirrels
Some CCHFV-transmitting tick species, like D. marginatus, occasionally feed on ground squirrels and hedgehogs. These mammals are suitable hosts for the instar life stages of many tick species, as they spend significant time rummaging through leaf litter and brush where immature ticks are present. Two types of ground squirrels have been experimentally infected, and findings were comparable in both studies.
3.2.1. Former Soviet Union
Blagoveshchenskaya et al. (1972) infected fifty 1–1.5-month-old susliks (Spermophilus pygmaeus) intracranially (IC), or IM and SC with strains Sudarkina and Hp-15 Zhr (37). Clinical signs were reported as absent in “most” animals, with no further details provided. Irrespectively of inoculation method, only slight immunomorphological changes were observed in the reticulohistocytic system, mainly lymph nodes and spleen, with peak changes observed 5–7 dpi. These organs appeared normal after 30–40 dpi, and morphological changes were absent in other organs and tissues. CCHFV was detected in the blood and parenchymal organs of all animals 2–7 dpi, but not later. Blood and parenchymal organ suspensions (diluted to 10−2) caused specific disease and death of NWM (37).
3.2.2. Africa
Shepherd et al. (1989) infected Cape ground squirrels (Xerus inauris) with SPU 4/81 strain. Viremia began at 2–8 dpi and lasted 7 days. Anti-CCHFV antibodies were detected 8 dpi by reverse passive hemagglutination inhibition (RPHI), with maximum titer detected 512 dpi (36).
3.3. Hedgehogs
In contrast to studies of ground squirrels, experimental studies on the susceptibility of hedgehog species to CCHFV yielded varying results, suggesting that susceptibility to CCHFV and infection dynamics may vary even between related species.
3.3.1. Former Soviet Union
In addition to hares, long-eared hedgehogs (Hemiechinus auritus) were also included by Zgurskaya et al. (1971) in early investigations of virus persistence in ticks (34). Hedgehogs were inoculated IV and IM as infection sources for Hyalomma ticks, but the magnitude of viremia was not reported (transmission studies detailed in Section 6.2). Blagoveshchenskaya et al. (1972) infected seven European hedgehogs (Erinaceus europaeus) and two long-eared hedgehogs (H. auritus) SC with strain Sudarkina, and no clinical signs were observed in any of the animals (38). Viremia was detected in H. auritus 2–6 dpi, peaking at 4–6 dpi, but no viremia was detected in E. europaeus. Noteworthy, H. auritus is common in Central Asia in regions endemic for CCHFV, while E. europaeus is found in Europe in regions that are generally free of CCHFV.
3.3.2. Africa
Shepherd et al. (1989) infected African hedgehogs (Atelerix frontalis) SC with strain SPU 4/81. Viremia was not detected, but anti-CCHFV antibodies were detected by RPHI starting 8 dpi and reaching a maximum titer at 256 dpi (36).
3.4. Small rodents
3.4.1. Africa
Shepherd et al. (1989) investigated infection in several small wild rodent species inoculated SC with 1 × 106–2.5 × 106 LD50 of strain SPU 4/81(36). Viremia ranging 3.2 × 101–1 × 104 LD50/mL and lasting 3–7 days was detected in white-tailed rats (Mystromys albicaudatus), red veld rats (Aethomys chrysophilus), bushveld gerbils (Gerbilliscus leucogaster), and four-striped grass mice (Rhabdomys pumilio). Viremia was not detected in highveld gerbils (Gerbilliscus brantsii), southern multimammate mice (Mastomys coucha), multimammate mice (Mastomys natalensis), and Namaqua gerbils (Desmodillus auricularis). Antibodies were detected in all species tested (sampled up to ≥ 35 dpi), with the exception of R. pumilio, which may have been due to limited sampling in that species (up to 8 dpi).
4. Experimental infection in livestock
Several CCHFV tick vectors feed on a variety of domestic animals, and may also be synanthropically associated with humans. Large domestic animals are known to support a high tick burden and bring ticks into close proximity to farmers. They can also expose humans to CCHFV directly through infected blood or crushing of engorged ticks on the animals during slaughtering (39–43). Seroepidemiological investigations have shown that a wide spectrum of domestic species is exposed to CCHFV (2), but experimental infection studies have been markedly more limited and have focused almost exclusively on assessment of viremia alone. Although small in number, experimental infections have been performed in several livestock species, including ruminants (cattle, sheep) and equids (horses, donkeys); these studies are summarized in Table 2.
Table 2. CCHFV experimental infection in livestock.
LD50 is indicative of NWM intracranial LD50 levels.
| Common name | Taxonomic name | Virus strain | Route | Viremia | Clinical signs | Antibody response | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cow | Bos taurus (White Fulani) (Mammalia/Artiodactyla) | IbAn 7620 (isolated from goat in this study) | SC (n = 1), SC/ID (n = 1) | Viremia 2 and 5 dpi in low dose inoculation; viremia 1–5 dpi in high dose inoculation | Yes, mild illness, dullness, lassitude, decreased appetite in both animals | NR | 1970 | (49) |
| Cow (calf) (2 mo, 6 mo) | Bos taurus (Mammalia/Artiodactyla) | Sudarkina | SC, IM, or SC/ID | Viremia | No | Yes | 1976 | (46) |
| Cow (calf) | Bos taurus (Mammalia/Artiodactyla) | K128-12, 3010, Nakiwogo, K67/67, IbAn 7620, JD 206, IbAr 10200 | IC and/or peripheral | Viremia (2 to 5–7 dpi, titer 1.6 × 104–1.6 × 106 LD50/mL)* | NR | Yes | 1979 | (48) |
| Sheep (Touabir and Peul-Peul breeds) | Ovis aries (Mammalia/Artiodactyla) | HD 49199 | IP or IT | Viremia (range 0–9 dpi) | Yes, fever (range 0–9 dpi), hepatic dysfunction, abnormal blood cell count | Yes, and passive transfer to offspring | 1998 | (45) |
| Sheep | Ovis aries (Mammalia/Artiodactyla) | SPU 4/81 | IT | 2–5 day duration (max 103 LD50/mL) | Mild fever | Yes | 1989 | (51) |
| Sheep | Ovis aries (Mammalia/Artiodactyla) | K128-12, 3010, Nakiwogo, K67/67, IbAn 7620, JD 206, IbAr 10200 | IC and/or peripheral | Viremia (day 2 to 5–7 dpi, titer 1.6 × 104–1.6 × 106 LD50/mL)* | NR | Yes | 1979 | (48) |
| Sheep (Peul-Peul Fulani breed) | Ovis aries (Mammalia/Artiodactyla) | Dak H49199 | IP | Viremia (0–6 days), corresponding to previous CCHFV exposure | NR | Yes | 1991 | (52) |
| Sheep (lamb) | Ovis aries (Mammalia/Artiodactyla) | Sudarkina | SC/ID | Viremia | No | Yes | 1976 | (46) |
| Donkey | Equus asinus (Mammalia/Perissodactyla) | NR | SC | Viremia (low level, titer NR) | No | Yes | 1972 | (53) |
| Donkey | Equus asinus (Mammalia/Perissodactyla) | K128-12, 3010, Nakiwogo, K67/67, IbAn 7620, JD 206, IbAr 10200 | IC and/or peripheral | Viremia (day 2 to 5–7 dpi, titer 1.4 × 104–1.6 × 106 LD50)* | NR | Yes | 1979 | (48) |
| Horse | Equus caballus (Mammalia/Perissodactyla) | Sudarkina | IV/IM or IM/SC | Viremia (low level, titer NR) | No† | Yes, high NAb | 1969 | (44) |
Abbreviations: dpi, days post infection; IC, intracranial; ID, intradermal; IM, intramuscular; IP, intraperitoneal; IT, inoculated ticks; IV, intravenous; NAb, neutralizing antibody; NR, not reported; SC, subcutaneous.
Clinical signs associated with inoculum.
Range for all species with detectable viremia; titer in individual species not reported.
Similarly to small mammals, livestock and equids develop a transient viremia and anti-CCHFV antibodies ~1 week post-inoculation. Additionally, maternal transfer of anti-CCHFV antibodies was demonstrated in sheep. These studies also further support the absence of clinical signs in the majority of animals tested, with the exception of sheep and horses. In the horse study, due to the nature of the inoculum, clinical signs (fever) were most likely associated with non-specific reaction to the inoculum matrix rather than virus infection (44). Whether CCHFV infection or the inoculum caused the fever in sheep is less clear, as the febrile period coincided with the viremic period (45). While these data help to provide a better understanding of infection in domestic species, they each include only a small number of animals, providing challenges for interpretation, and must be broadly considered.
4.1. Cattle
4.1.1. Former Soviet Union
Zarubinsky et al. (1976) experimentally infected calves with the Sudarkina strain (46). Two 6-month-old calves were inoculated either IM or SC, and two 2-month-old calves were inoculated both SC and ID. Clinical disease was not observed in any of the animals, and viremia was not detected in the 6-month-old calves; however, CCHFV was isolated from the blood of one of the 2-month-old calves at 3 and 7 dpi, supporting the possibility of virus transmission to humans from exposure to blood of infected animals (18–20). Anti-CCHFV antibodies, detected by agar-gel diffusion precipitation (AGDP) performed 5–20 dpi in samples from the 6-month-old calves, were seen at 13 and 17 dpi in the SC-infected and the IM-infected calf, respectively; antibodies were detected by CF only in the IM-infected animal (17 dpi). Additionally, antibodies were investigated in the SC/ID inoculated 2-month-old calves using the indirect hemagglutination inhibition test 5–35 dpi (47); antibody titers of 1:10 were detected in 1 calf on days 9, 14, and 20 after infection, increasing to 1:80 at 35 dpi. Smirnova (1979) inoculated calves with a variety of strains of CCHFV (48). As described for other animals tested (Section 4.2.1, 4.3.1), calves developed antibodies detectable by AGDP and CF, and viremia was observed at 2 to 5–7 days (viremic period reported collectively for all species tested).
4.1.2. Africa
In 1970, Causey et al. isolated CCHFV from cattle, a goat, and a hedgehog. Two White Fulani (Bunaji) breed calves, 9 and 10 months of age, were inoculated with the goat isolate (IbAn 7620) (49). One calf was inoculated SC with 2.0 × 108 LD50 (low dose animal), and the other calf was inoculated both SC (2.0 × 108 LD50) and ID (1.3 × 107 LD50) (dual route, high dose animal). Five days prior to inoculation, feeding capsules of laboratory-reared Hyalomma rufipes ticks were attached to each animal (transmission data further discussed in Section 6.2). Prior to the study, ticks and calves all tested negative for CCHFV and neutralizing antibodies to CCHFV, respectively. After inoculation, both calves developed mild illness, characterized by dullness, lassitude, and decreased appetite. Viremia was detected 2 and 5 dpi in the low-dose calf, and 1–5 dpi in the high-dose calf. Viremia was determined by inoculation of suckling mice, and CCHFV presence was confirmed by compliment fixation (49).
Shepherd et al. (1991) inoculated two cows SC with CCHFV strain SPU 4/81 to assess transmission to ticks. The cows were viremic 5–8 dpi or on 6 dpi. In accordance with the previous studies, no clinical signs were reported (50). Tick transmission data are reviewed below (Section 6.2).
4.2. Sheep
4.2.1. Former Soviet Union
Kondratenko et al. (1968) identified cattle, sheep, and goats as the main hosts of adult ixodid tick vectors of CCHFV in Rostov Oblast (Kondratenko et al. 1968, in [46]). Thus, in addition to experimental infection of cattle (see 4.1), Zarubinsky et al. (1976) experimentally infected five lambs (2–2.5 months old) SC and ID with the Sudarkina strain (46). No clinical disease was observed in any of the animals, but CCHFV was isolated from the blood of all lambs (4–8 dpi in lamb 1; 4–5 dpi in lamb 2; 6–8 dpi in lamb 3; 4–6 dpi in lamb 4; and 4–7 dpi in lamb 5). AGDP analysis of lamb sera 5–35 dpi indicated the presence of precipitating antibodies at 21 dpi (lambs 1, 2, 3, and 5) and 35 dpi (lamb 4). Smirnova (1979) inoculated sheep with various CCHFV strains (48). As described for other animals tested (Section 4.1.1, 4.3.1), sheep developed antibodies detectable by AGDP and CF, and viremia was observed at 2 to 5–7 days (viremic period reported collectively for all species tested, so exact timing for each species is unclear).
4.2.1. Africa
To understand the impact of infection on sheep and their ability to replicate CCHFV, Gonzalez et al. (1998) infected 10 adult (> 1 year old) naïve sheep intraperitoneally (IP; n = 2) or via CCHFV-infected Hyalomma truncatum ticks (n = 8) with CCHFV strain HD 49199 (45). Fever (39.7°C ± 0.3) was observed in the sheep beginning at 3 dpi, peaking around 1°C above normal for 5 days and declining within 36 h thereafter. Viremia was detected in all infected animals and correlated with fever, starting at 3 dpi, peaking at 5 dpi (5.0 × 104 LD50/mL), and lasting 4–7 days total. Interestingly, no differences were noted between IP- and tick bite-infected sheep. Renal and liver functions were assessed in a subset of the infected sheep; in all sheep, a slight but significant increase was observed in aspartate aminotransferase levels for 3 to 10 days starting at 3 dpi, but no changes were observed in azotemia, creatinemia, or alanine aminotransferase. Five of the infected sheep were pregnant ewes, of which four normally delivered healthy lambs and one spontaneously aborted. No virus was isolated from the aborted fetus, suggesting unrelated etiology of the abortion. Enzyme-linked immunosorbent assays (ELISA) of non-immune sheep samples showed an IgM response 7 dpi, followed by an IgG response one day later.
In studies investigating ticks species as potential vectors of CCHFV (described in Section 6.1), Shepherd et al. (1989) reported that sheep inoculated via infected ticks developed mild fever and a low viremia (max intensity 103 LD50/mL) lasting 2–5 days, followed by the development of antibodies detectable by immunofluorescence (IF) (51).
Wilson et al. (1991) used IP inoculation of 3.2 × 105 LD50 of strain Dak H49199 to challenge previously exposed, maternally exposed, and naïve sheep with varying levels of previous exposure to CCHFV (52). The group found that viremia corresponded to previous exposure: naïve-infected sheep in the study developed the highest and most prolonged viremia detected 2–6 dpi, and high titers of IgM beginning 6 dpi, followed by elevated IgG. Similar patterns of viremia to those in naïve-infected animals were reported in a separate study by Shepherd et al. (1991) in sheep [n = 3] inoculated SC with SPU 4/81; in that study, viremia was detected 2–6 dpi or 4–7 dpi (50). To test the effects of intermediate CCHFV exposure, Wilson et al. used the offspring of a previously exposed sheep; the offspring had weak pre-infection IgG as a result of maternal transfer. After experimental infection, this offspring developed low viremia 3–6 dpi, weakly elevated IgM beginning 9 dpi, and IgG levels that slowly increased over the study period. The sheep that had been previously inoculated had high levels of CCHFV IgG prior to re-challenge. Re-challenge resulted in undetectable viremia, slightly elevated IgM titers, and persistence of high levels of IgG. Notably, despite the absence of detectable viremia in sheep with previous infection, CCHFV was transmitted to 11% (n = 18) of female ticks feeding on these sheep (52).
4.3. Equids
4.3.1. Former Soviet Union
The role of equids in maintenance and transmission of CCHFV is not well described, but serological data support natural exposure to virus (2). Experimental infection studies were performed in both horses and donkeys to investigate the possibility of producing antibodies for putative antibody-based human therapeutics. Milyutin et al. (1969) infected two 3-year-old mares IV and IM, or IM and SC, with CCHFV strain Sudarkina. Hyperthermia as high as 40.3°C, somnolence, and weakness were observed at 1–2 dpi, before the animals’ condition normalized (44). Increased erythrocyte sedimentation rate, indicating non-specific inflammation, and an insignificant leukocytosis were observed. No infectious virus could be detected in blood or urine at 2 dpi or at any later time points investigated (3–7, 9, 11, 13, 15, and 20 dpi). Modest serological response was detected by AGDP, with titers up to 1:32 in the CF assay (44).
Based on concerns about anaphylactic reactions in humans to antibodies produced from horse blood sera, Rabinovich et al. (1972) subsequently investigated anti-CCHFV antibody production for therapeutic use in donkeys via experimental infection (53). Two donkeys (4 and 5 years old) were inoculated SC with 9.5 × 108 LD50 of CCHFV (strain not reported). Animals were observed for 10 dpi, and no changes in temperature or blood analysis parameters were detected. Low-level viremia was observed 4, 6, and 7 dpi, indicated by “some” disease in NWM (titer was not reported). CF detected anti-CCHFV antibodies in the donkeys at 8 and 13 dpi. Antibody titers increased after a second inoculation of the donkeys 15 days after the primary inoculation. Neutralizing activity of the serum from donkeys was comparable to the activity observed in sera from inoculated horses, but the report does not make clear whether the donkey sera were collected after the initial or the secondary inoculation. No precipitating antibodies were detected (53). Smirnova (1979) inoculated donkeys with several CCHFV strains (48). As described for other animals tested (Section 4.1.1, 4.2.1), donkeys developed antibodies detectable by precipitation and CF, and viremia was seen 2 to 5–7 dpi (viremic period reported collectively for all species tested).
5. Experimental infection in birds
The role of birds is the least well-understood aspect of CCHFV maintenance and transmission. Despite extensive investigations, the absence of serological evidence of CCHFV-specific antibodies in most avian species sampled suggests that most birds are predominantly refractory to CCHFV infection. However, resistance is not uniform, as there is serological evidence of exposure in some species (2). In the original outbreak in Crimea in 1944, Grobov collected immature H. marginatum ticks from wild birds and domestic fowl, considering birds as the putative reservoir of the then-unknown infectious agent (54). Birds were suspected as important in virus maintenance due to their extremely high tick infestation rates and infestation indexes (calculated as the [no. ticks/no. infested animals] × [% of infected animals]). The infestation index of immature H. marginatum in eight species of ground-feeding birds in the Volga River delta (collected 1963–1964) ranged 3.3–68.9 (55). The highest infestation index was in rooks (Corvus frugilegus); hundreds of H. marginatum have been noted to feed on a single rook in CCHFV-endemic regions (56). Indeed, CCHF cases in the Volga River floodplains and delta (Astrakhan and Rostov Oblasts) epidemiologically linked birds, specifically tree-roosting C. frugilegus hosting immature H. marginatum ticks near cattle pastures, to disease occurrence (1). In addition, virus has been isolated from ticks feeding on birds (57).
Overall, reports to date show that birds may be important tick blood meal hosts implicated in wide-range transportation of tick reservoirs, but very rarely in CCHFV amplification (Table 3). The absence of detectable viremia was reported in experimental studies of both wild and domestic avian species, with ostriches as a notable exception, emphasizing the importance of not overgeneralizing CCHFV infection dynamics in animal taxa. Ostriches can host a variety of tick species involved in CCHFV transmission (1), develop detectable and sustained viremia, and have been epidemiologically associated with human infection (58).
Table 3. CCHFV experimental infection in birds.
Reports of experimental infection in birds are limited to a few independent investigations. Viremia was undetectable in most species, with the notable exceptions of guinea fowl and ostriches. Clinical signs were not reported in any animals.
| Common name | Taxonomic name | Virus strain | Route | Viremia | Antibody response | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Red-billed hornbill | Tockus sp. (Aves/Bucerotiformes) | HD 49199 | IP | No viremia detected, but transmitted virus to naïve ticks | Yes | 1994 | (67) |
| Laughing dove | Spilopelia senegalensis (Aves/Columbiformes) | HD 49199 | IP | No viremia | No | 1994 | (67) |
| Rock dove | Columba livia (Aves/Columbiformes) | NR | NR | No viremia | No | 1969–1972 | (61–63) |
| Chicken | Gallus gallus (Aves/Galliformes) | HD 49199 | IP | No viremia | Yes (in 1 of 6 animals) | 1994 | (67) |
| Chicken | Gallus gallus (Aves/Galliformes) | SPU 4/81 | SC/IM/IV | No viremia | No | 1987 | (64) |
| Blue-helmeted guinea fowl | Numida meleagris (Aves/Galliformes) | SPU 4/81 | SC/IM/IV | 3-week-old and adult birds similar, low-level viremia in 10–20% of animals; most refractory | Yes, beginning 5–6 dpi | 1987 | (64) |
| Glossy starling | Lamprotornis sp. (Aves/Passeriformes) | HD 49199 | IP | No viremia | Yes | 1994 | (67) |
| Rook | Corvus frugilegus (Aves/Passeriformes) | NR | NR | No viremia | No | 1969–1972 | (61–63) |
| Ostrich | Struthio camelus (Aves/Struthioniformes) | SPU 4/81 | SC | Viremia 1–4 dpi (max 1 × 104 LD50/mL at 2 dpi); virus detected in spleen, liver, kidney (up to 5 dpi) | Yes | 1998 | (69) |
Abbreviations: dpi, days post infection; IM, intramuscular; IP, intraperitoneal; IV, intravenous; NR, not reported; SC, subcutaneous.
Birds that do not develop viremia likely play a role in the introduction of infected ticks into new territories by carrying feeding immatures that were transovarially infected from adult ticks. The role of birds in CCHFV maintenance and transmission may be more extensive. Anti-CCHFV antibody production has been reported independently of detectable viremia, and in addition to ostriches, persistent anti-CCHFV antibodies were detected in a red-beaked hornbill (Tockus sp.) and glossy starling (Lamprotornis sp.). Furthermore, while viremia may have been missed due to assay sensitivity or timing issues, studies have shown that CCHFV or tick-borne encephalitis virus can be transmitted from one co-feeding tick to the next in the absence of detectable viremia in the host (9,59,60). The concept of threshold viremia therefore might not apply to tick-borne viruses and might not be the hallmark feature for identifying hosts involved in CCHFV maintenance.
5.1. Former Soviet Union
The earliest reported experimental investigations into the role of birds were performed by Berezin et al. (1969 – 1971), but never published (as described in [1]). Rooks (C. frugilegus) and rock doves (Columba livia) were experimentally inoculated with CCHFV. No clinical signs were observed, no virus was isolated, and no serological evidence of viremia was found (data reviewed in [61–63]).
5.2. Africa
In later investigations to assess viral pathogenicity in wild birds, Shepherd et al. (1987) inoculated six adult and 20 3-week-old blue-helmeted guinea fowl (Numida meleagris) with strain SPU 4/81, and measured viremia and antibody responses (64). Adult guinea fowl were inoculated SC, IM, and IV (1.5 × 107 LD50 total); 3-week-old guinea fowl were inoculated IM and SC (5 × 106 LD50 total). Viremia was assessed 1–9 dpi, and was detected only intermittently in a small subset of the adult and 3-week-old animals (0–20% of the birds tested per day). Most of the young birds were refractory to infection based on antibody assessment: antibodies were detected in 8 of 20 serum samples taken 7–21 dpi, and in 5 of 16 birds bled on day 28. Antibodies were detected by IF in adult birds starting 5 dpi, and by RPHI on day 6 after infection; maximum titers were observed 8 and 9 dpi, and RPHI and IF antibody titers dropped to undetectable levels by 49 dpi in most of the experimentally infected guinea fowl. These studies highlighted the transient nature of the antibody response in birds, and by using a variety of techniques (immunodiffusion, RPHI, and IF) to detect antibodies, they demonstrated that the immunodiffusion test is unsuitable for use in serological surveys to detect CCHFV antibodies in birds (64–66).
Zeller et al. (1994) experimentally inoculated laughing doves (Spilopelia senegalensis; n = 2), a red-beaked hornbill (Tockus sp.; n = 1), and a glossy starling (Lamprotornis sp.; n = 1) IP with strain HD 49199 (3.2 × 101–3.2 × 103 LD50) (67). Viremia was not detected in any of the birds from 2–10 dpi, though the glossy starling was not tested. Antibodies to CCHFV were not detected in the laughing doves, but a significant antibody response was seen in the red-beaked hornbill and the glossy starling despite the absence of detectable viremia. Anti-CCHFV antibodies were still detectable in these birds 4 months later (67). This suggests that the existing assays were unable to detect viremia in these species, that viremia was acutely transient, or that antibody development may occur in the absence of detectable viremia.
The embryonated chicken egg has been successfully infected and used for propagation of CCHFV (68); experimentally infected chickens (Gallus gallus domesticus) do not appear susceptible to infection. In parallel to inoculations in guinea fowl (see above), Shepherd et al. (1987) inoculated 6 adult chickens, detecting no viremia and no antibodies by RPHI test in sera collected up to 28 dpi (64). Zeller et al. (1994) inoculated 6 chickens IP with a series of challenge doses of CCHFV strain HD 49199 (3.2 × 103 LD50, n = 4; 3.2 × 102 LD50, n = 1; 3.2 × 101 LD50, n = 1). Again, viremia was not detected in any of the animals 2–10 dpi, and antibody-capture ELISA detected no anti-CCHFV antibodies (67).
While chickens appear refractory to infection, studies by Swanepoel et al. (1998), following an outbreak of CCHF in workers on an ostrich farm (58), reported viremia 1–4 dpi (maximum at 2 dpi) in experimentally infected ostriches. Anti-CCHFV antibodies were detected by compliment ELISA in 1 of 9 ostriches at 5 dpi, and by 13 dpi all animals had seroconverted. CCHFV was detected in spleen, liver, and kidneys up to 5 dpi, 1 day after it was no longer detectable in blood. No infective virus was detected in muscle samples, but viral RNA was detected by reverse transcription-polymerase chain reaction (RT-PCR) in muscle from an ostrich sacrificed on day 3 pi (69).
6. Experimental vector transmission studies
CCHFV is only transiently associated with the vertebrate host but persists in ticks; mammals remain viremic for no more than a few weeks (usually ~ 1 week), but ticks undergo life-long infection. As a result, ticks are considered the vector and the long-term reservoir for the virus in nature (1,70–72). Inoculation route may affect resultant viremia; ID inoculation, which most closely mirrors tick transmission, resulted in highest viremia levels in hares (35). In addition, characteristics of the vector may influence transmission efficiency. Compared to mosquitoes, ticks feed for a long period of time and need to immunomodulate the bite site using biologically active molecules secreted in tick saliva. Tick-borne pathogens have been known to exploit these mechanisms. Certain molecules in arthropod saliva have been shown to promote pathogen transmission to the vertebrate host, a phenomenon termed saliva-assisted transmission (SAT). SAT is well documented in transmission of several tick-borne pathogens (73–75), but has only been demonstrated in two CCHFV studies (67,76). SAT factors appear to differ among tick vector species, and might depend on the vertebrate host species on which the tick feeds, indicating the need for the right tick-host pairing in experimental studies. Additionally, SAT can lead to higher viremia levels in infected animals than produced by traditional inoculation, further emphasizing the importance of using true vector transmission in experiments in addition to traditional needle inoculation. Elucidating the properties of circulation and preservation of CCHFV in its natural foci and the possibility of transstadial and transovarial transmission in ticks was prioritized in early experimental studies. Several studies have investigated CCHFV infection via tick inoculum and virus transmission to ticks from inoculated animals.
6.1. Transmission from ticks to vertebrates
Transmission of CCHFV from infected ticks to mammals was investigated in cattle, sheep, and guinea pigs. Initial reports of tick transmission by Lee and Kemp were based on limited experimental infection studies in calves. Susceptible 6-month-old (~100 kg) White Fulani calves (n = 2) were inoculated using adult H. rufipes ticks that were infected with strain IbAn 7620 as nymphs (77). The inoculation process was noted to cause trauma to the ticks; considerable seepage of body fluid and inoculum from the puncture sites was observed, and it was unknown what amount of virus suspension was actually administered, or to what tissue sites. Although testing subsets of the ticks indicated low infection success, 11 of the inoculated ticks were placed on one calf and left to feed for 14 days; viremia was detected in the calf on days 2 and 7 post exposure. In addition, progeny of one female tick that fed on this calf contained viable CCHFV, supporting vertical CCHFV transmission in ticks. The second calf was subsequently used to examine transmission from larvae of an adult female tick that had fed on the first calf. This female tick was allowed to feed on a rabbit and molt before being placed onto the second calf, which did not develop viremia. However, while virus was detected in larvae from the infected female tick, virus was not confirmed in any of the nymphs tested, so little can be concluded from the absence of viremia in the second calf (77).
Shepherd et al. (1989) intracoelomically infected groups of adult H. rufipes, H. truncatum, and Rhipicephalus evertsi mimeticus with strain SPU 4/81, and allowed the ticks to feed on susceptible sheep 56–97 days later. CCHFV was successfully transmitted to the sheep (51). Later, four pools of larvae from each of the above species of infected ticks above were allowed to feed on susceptible guinea pigs (51), and 1,357 larvae were subsequently removed from the guinea pigs. Virus was not isolated from the larvae, and anti-CCHFV antibodies were not detected in the sera of guinea pigs collected 21 dpi. Although transstadial and transovarial transmission have been reported for these species (H. rufipes [77]; H. truncatum [51,52]; R. evertsi mimeticus [51]), the lack of virus in the larvae suggests that transovarial transmission did not occur in these studies and the larvae used were likely not infected, thus prohibiting assessment of CCHFV transmission by ticks to the guinea pigs in this report. Tick transmission may not be well modeled via experimental (intracoelomical) infection, as artificial infection did not lead to detectable infection in progeny, whereas natural infection (i.e., by allowing ticks to feed on infected vertebrates) in studies described below do (see 6.2). This supports the influence of tick host factors on transmission that is not modeled by some experimental approaches.
While not demonstrated by Shepherd et al. (51), transmission from ticks to guinea pigs does occur. This was shown in earlier studies by Berezin et al. (1971), who investigated CCHFV persistence in H. marginatum ticks at 4°C (78). Ticks maintained for 7–9 months at low temperatures were able to transmit CCHFV to guinea pigs; the hosts developed viremia 11 and 12 days post attachment, and antibodies were detected in surviving animals 21 days following tick bites, further supporting long-term CCHFV persistence in the tick vector. Notably, in these animals the infection course was described as severe and frequently fatal (78), whereas clinical signs have not been reported in other studies of experimental infection of guinea pigs (48). Thus, transmission via natural tick vector rather than by needle inoculation may produce more severe illness; experimental infection by tick transmission should therefore be considered in CCHFV studies.
6.2. Transmission from vertebrates to ticks
Experimental transmission from inoculated animals to ticks has been demonstrated in a variety of species: cattle, sheep, susliks (S. pygmaeus), long-eared hedgehogs (H. auritus), hares (L. saxatilis), and laboratory rabbits. Causey et al. (1970; see Section 4.1) investigated CCHFV transmission from cattle to ticks by placing H. rufipes ticks on two calves that were inoculated with IbAn 7620 five days after tick placement. One of the calves developed a local purulent reaction at the feeding site. The second calf supported the tick infestation well: tick feeding was observed 1 day prior to inoculation. A single engorged female dropped off 11 dpi, oviposited 4 days later, and was positive for CCHFV 18 days after dropping (24 days after viremia was last detected in the calf). Virus was not detected in the resulting larvae tested 27 days after oviposition (49).
Early investigations suspected the prominent role of ticks as the CCHFV reservoir, and animals were often used in experimental studies for understanding CCHFV maintenance in the tick while tangentially providing data on transmission from animals. Zgurskaya et al. (1971) were interested in interepidemic virus survival via transovarial and transstadial transmission in ticks (34). European hares (L. europaeus) and long-eared hedgehogs (H. auritus), inoculated IV and IM, were found to be susceptible to experimental CCHFV infection and were used as CCHFV infection sources for H. marginatum ticks (See Sections 3.1.1. and 3.3.1.). Virus was detected in nymphs collected from both L. europaeus and H. auritus, and from adult ticks that molted from nymphs, confirming transmission from animals to ticks and transstadial transmission in H. marginatum ticks. The group went on to use animals to confirm that H. marginatum can preserve CCHFV following artificial overwintering conditions (45 days at 4°C) for 13 months, and to demonstrate transovarial transmission by confirming infection (confirmed by antibody detection) via transmission in guinea pigs and rabbits (34). Blagoveshchenskaya et al. (1975) also investigated transmission from infected hedgehog to ticks (38). After confirming hedgehog susceptibility to CCHFV (see Section 3), they demonstrated that the virus could be transmitted from H. auritus to H. marginatum ticks, but not to Rhipicephalus rossicus or D. marginatus ticks (38).
Later studies by Zgurskaya et al. (1975) investigated threshold levels of CCHFV for infecting H. marginatum by allowing ticks to feed on infected lagomorphs (hares, rabbits) (79). Animals were inoculated IV and IM with CCHFV (strain not reported). Viremia was determined in the animals and related to infection in associated blood-fed ticks. Threshold levels for CCHFV transmission from the lagomorphs to the ticks were estimated to be 2.5 × 104–2.0 × 105 LD50/mL; animals that developed this range of viremia (n = 3) transmitted CCHFV to ticks, while no transmission was observed from animals (n = 2) with a viremia of 6.3 × 103–2.5 × 104 LD50/mL. These studies were limited to lagomorphs and investigated few animals and ticks, but highlight an important principle in maintenance and transmission that warrants additional investigation.
Zarubinsky et al. (1976) placed laboratory-reared H. rufipes, R. rossicus, and D. marginatus ticks on 2 experimentally infected 6-month-old calves 24 h after the calves were infected with CCHFV. No ticks became infected from feeding, but no viremia was detected in the calves, prohibiting any conclusions to be drawn from these investigations (46). However, a subsequent study of ticks that fed on viremic cattle infected with strain SPU 4/81 (50) confirmed transmission of CCHFV to adult ticks of five species (H. truncatum, Rhipicephalus appendiculatus, R. e. evertsi, R. decoloratus, and Amblyomma hebraeum); transmission to H. rufipes was not detected.
Additional transmission studies in livestock by Wilson et al. (1991) demonstrated that sheep may serve as amplification hosts for CCHFV, and that their relative involvement is influenced by prior exposure status. Female West African Peul-Peul sheep (n = 4) were infested with 30 male and 30 female H. truncatum ticks 2 days prior to CCHFV inoculation. Three sheep were then inoculated IP with 3.2 × 105 LD50 of CCHFV strain Dak H49199, and 1 sheep was used as an uninfected control. Beginning at 4 dpi, ticks were examined daily; engorged females that had naturally finished feeding were removed, and male ticks were detached by forceps at 16 dpi. Virus was detected in at least one tick that fed on each of the three inoculated sheep. Higher viremia corresponded to lower levels of previous exposure of the sheep to CCHFV and to lower levels of previously acquired anti-CCHFV antibodies. Virus was detected in 11% of the female and 0% of the male ticks collected from the previously exposed sheep with undetectable viremia (previously described in 4.2.2); in 14% of the female and 30% of the male ticks from the weakly viremic offspring of a previously exposed sheep; and in 33% of the female and 60% of the male ticks from strongly viremic sheep infected for the first time. Time of detectable viremia in the sheep corresponded to the time during which infected female ticks were detaching. Transovarial transmission was detected in two of 12 (17%) of egg batches from infected female ticks (52).
In a study by Shepherd et al. (1991), three sheep inoculated SC with a different human isolate (strain SPU 4/81) were fed on by adult H. truncatum (as in the previous study), A. hebraeum, H. rufipes, R. e. evertsi, R. appendiculatus, and Rhipicephalus simus ticks, and by larval or nymphal A. hebraeum, R. e. evertsi, and R. simus ticks. Four hundred and forty-three pools of ticks were tested (adults; immatures; females after oviposition, and larvae from these eggs; and adults that had molted from engorged immatures); none yielded CCHFV despite detectable viremia (3.2 × 102 –1.6 × 103 LD50/mL) in the sheep (50).
Shepherd et al. (1991) conducted additional transmission studies in small mammals; no CCHFV was isolated from engorged larvae of A. hebraeum, H. truncatum, or R. e. mimeticus that dropped off infected white rats (M. albicaudatus), nor from nymphs or adults of these species tested after molting (50). Similarly, no CCHFV was isolated from engorged A. hebraeum, H. truncatum, or R. e. mimeticus larvae that fed on three infected Duncan-Hartley guinea pigs, nor in A. hebraeum or H. truncatum nymphs tested after molting (50). These studies suggest that the tick-host interaction is complex, and only certain combinations of hosts and ticks support CCHFV transmission.
6.3. Tick-borne transmission from vertebrate to vertebrate
In 1970–1971, Levi and Vasilenko investigated circulation and maintenance of CCHFV in the tick vector, finding evidence of transovarial transmission in Hyalomma plumbeum ticks and demonstrating the full vertebrate-tick-vertebrate transmission cycle. Ticks were fed on Belgian giant breed rabbits (Oryctolagus cuniculus) inoculated IV with CCHFV-infected plasma of living animals and high-titer liver suspensions, defined as ~3.2 × 104–3.2 × 105 LD50 (the CCHFV strain and animal sources of plasma and liver suspensions were not reported). After 10 days (for adults) or 20 days (for larvae or nymphs) of feeding, the ticks were collected and tested immediately or in later stages of development. CCHFV was detected in all fed ticks and their offspring, confirming transstadial and transovarial transmission. Additionally, transovarially infected nymphs were placed on uninfected naïve rabbits that subsequently developed AGDP-detectable antibodies to CCHFV, with antibody titers positively correlating with infected tick burden (80).
In 1976, Kondratenko et al. investigated the importance of ixodid ticks in CCHFV transmission and maintenance by feeding 50–500 ticks (H. plumbeum, R. rossicus, or D. marginatus) on each of six susliks (S. pygmaeus; 2–3 months old) infected SC with a NWM brain suspension of strain Sudarkina (56 passages [81]). Subsets of engorged nymphs were tested; others were kept at 22–25°C and 60–80% humidity and allowed to molt to adults. Adults were maintained at 4°C, and tested every 1–2 months for CCHFV. Following artificial overwintering, adults were fed on 1.5–2 months old naïve susliks (Spermophilus sp.) or 6-week-old rabbits to investigate infection transmission to vertebrates and transovarial transmission in ticks. Ticks infected with CCHFV, irrespective of the developmental stage at feeding, transmitted virus to susliks and rabbits by feeding on them within 620 days (observation period) of initial infection.
Transmission between vertebrates via tick vector was later demonstrated in additional studies. Shepherd et al. (1991) demonstrated a cycle of hare-tick-sheep virus transmission; scrub hares (L. saxatilis) infected with SPU 4/81 transmitted CCHFV to H. truncatum and H. rufipes ticks, and sheep on which these ticks subsequently fed seroconverted (50). Notably, Zeller et al. (1994) reported CCHFV transmission from birds to ticks and then subsequent transmission from ticks to rabbits (67), despite extensive serological data suggesting that birds are refractory to infection (2). Experimental transmission studies followed initial experimental infections of birds (Section 5), and were performed using three red-beaked hornbills (Tockus sp.), three glossy starling (Lamprotornis sp.), and three domestic chickens (G. g. domesticus) by placing H. rufipes larvae on the head of each bird. The birds were inoculated with CCHFV at different points after larvae placement: same day (1 chicken), 3 days later (2 chickens, 2 hornbills, and 1 starling) to assess CCHFV transmission to larvae, or 10 days later (1 chicken and 1 hornbill) to assess transmission to nymphs. Virus isolation was performed on engorged nymphs that had dropped off the birds and were collected and allowed to molt. Adult ticks derived from some of the molted nymphs were then allowed to feed on rabbits. Male and female adult ticks and progeny were tested for CCHFV, and rabbits were tested for anti-CCHFV antibodies. Chickens did not transmit the virus to H. rufipes larvae or nymphs. Larvae placed on the hornbills and starling did not drop off the birds after engorgement, but molted on the host and remained attached until the engorged nymph stage (≥ 2 weeks duration). Two of the hornbills died 14 dpi, likely due to heavy tick infestation (> 150 nymphs). Nymphs were collected from the cadavers and virus was isolated from all 17 pools of 10 nymphs each. Anti-CCHFV antibodies were detected in one hornbill and the starling. The adult ticks emerging from molted nymphs derived from the hornbills and starling transmitted CCHFV to rabbits, inducing a detectable antibody response (67).
Transmissions from ticks to vertebrates and from vertebrates to ticks, as well as a full vertebrate-tick-vertebrate transmission cycle, have been recapitulated in experimental studies. While results were often variable (e.g., tick transmission to 1 of 2 calves or sheep tested), these studies provide important proof of principle for the widely accepted viral enzootic cycle in nature, and challenge evidence suggesting a limited role of birds as susceptible hosts in CCHFV maintenance. Importantly, these limited data also suggest potential limitations of using traditional lab species in transmission studies, as rats and guinea pigs did not transmit virus to ticks in the laboratory setting.
7. What we have learned
Nearly 40 years after the publication of Hoogstraal’s landmark monograph (1), many aspects of the complex relationship between CCHFV, mammalian hosts, and tick vectors still need to be elucidated. Clearly understanding the transmission and maintenance cycle of CCHFV is not a simple task, as CCHFV is widely disseminated, with a multitude of vertebrate and arthropod species involved. Importantly, however, we do broadly understand the major ecological contributors to CCHFV maintenance and transmission necessary for general public health education, clinical awareness, and intervention strategy development (e.g., acaricide use).
Similar to interpretation of serological studies, where serology does not seem to adequately correlate with viremia or with the ability of the seropositive animal to infect feeding ticks, experimental infection data must not be over-interpreted. Limited data from experimental infections, without considering the full spectrum of ecological contributors like tick-host dynamics, may not accurately reflect relative roles of the tested animal in a natural transmission setting.
Despite limitations, experimental animal studies have been critical in providing CCHFV data to complement the numerous serological studies performed in animals, and provide key insights into all aspects of the hypothesized CCHFV transmission cycle. Most mammals appear susceptible to infection, as indicated by detection of anti-CCHFV antibodies and/or viremia. However, despite CCHFV isolation from mammalian species, a prominent role for mammals as long-term reservoirs of CCHFV is not supported due to brief and transient viremia. This is further supported by the limited reports of virus isolation from mammals compared to isolation from ticks. As such, these studies support ticks as reservoirs for viral persistence in nature, with vertebrates providing a necessary blood meal for the ticks and serving as potential amplification hosts.
8. What we still do not know
The current knowledge of major ecological contributors to maintenance and transmission of CCHFV is necessary for ongoing interventions, but not sufficient. Similar to many other zoonotic agents, CCHFV causes little or no disease in its livestock hosts, and thus is not typically a disease of veterinary concern. Nevertheless, CCHFV merits significant veterinary consideration, as animal hosts are essential for the tick vectors. Vertebrates have a critical role in viral maintenance; moreover, movement or transport of viremic animals, or animals bearing infected ticks, can introduce CCHFV into new geographic areas.
Viremia in vertebrates is short-lived, of variable intensity, and notably absent in several species tested in the laboratory (e.g., [45,46,50,52]). However, vertebrate-to-tick transmission is highly efficient and thus naturally regulated, in part, by the brevity of the viremic period in vertebrates. Even if Hyalomma genus tick larvae and nymphs feed consecutively on the same host without molting on the ground, the overlap between host viremia development and the feeding of ticks in different stages must be finely tuned for CCHFV transmission.
A narrow window of viremia may, however, be the origin of an amplification route. Initially it was proposed that adults were the only tick stage that overwinters (82). Subsequent studies have shown that both adults and developing eggs may overwinter (83). As different hibernating tick stages may be activated at different times of the year, the onset of annual activity of Hyalomma spp. larvae and nymphs may last for several weeks (83). Each spring, the larvae initiate the life cycle by feeding on new hosts after temperatures reach a threshold to activate them after hatching. The time of larval feeding to nymphal feeding on the same host may last between 20 and 30 days. Therefore, feeding nymphs may overlap on the same host with newly attached larvae. If the nymphs are infected and transmit the virus to the host, and the host develops viremia, newly acquired larvae could become heavily infected. Such a mechanism would require that the first groups of feeding larvae that develop into nymphs are transovarially infected, later spreading infection to newly acquired larvae that subsequently molt to nymphs. This hypothesis jointly considers the transovarial route of infection of the newly hatched larvae and the amplifying role of the hosts in infecting new larvae. The ability of the infected hosts to transmit CCHFV to ticks, and, therefore, the number of ticks infected from a single amplification host, is unknown. These data are relevant for considering interventions like acaricide treatment and vaccination of livestock. Although acaricide treatment of animals, e.g., before or during marketing, is thought the be an effective intervention strategy and is linked to decreased seroprevalence (84,85), efficacy has not been sufficiently investigated. Ultimately, for these and other putative intervention strategies (e.g., anti-tick vaccines (86–88) or anti-CCHFV vaccines [90,91]), proper development, evaluation, and implementation would require a more detailed understanding of tick-host interactions and viral transmission.
Animals are widely susceptible to CCHFV infection, but susceptibility is not universal among all animal species. Specifically, the vast majority of avian species investigated appear refractory to infection; serological studies report the absence of CCHFV antibodies in birds, and no viremia was detected in almost all experimentally infected avian species. There are two notable exceptions: low-level viremia was detected in blue-helmeted guinea fowl (N. meleagris) (64), and ostriches developed high-titer viremia and have been associated with human CCHFV cases (69). The role of birds in CCHFV maintenance and transmission is far from well understood. The existence of cryptic transmission cycles, the exchange of virus between two cycles when tick vectors feed on the same host (59,91), needs to be investigated in birds. How detectable viremia translates into a role in maintenance and transmission of CCHFV is unclear. Arbovirus transmission to ticks from non-viremic birds has been reported (92,93), and co-feeding is an efficient source of tick-to-tick transmission that is independent of detectable viremia in the host; this is a consideration for birds and all vertebrate hosts.
Considering the relatively low numbers of feeding immature ticks that birds can transport, the contribution of birds as amplification hosts seems to be low. Birds, however, may be a very effective method of transporting ticks during migratory flights, and appear to have a crucial role in distributing ticks over wide geographic areas (94). Considering the time immature ticks spend feeding on birds, birds probably do not directly transport infected ticks from an origin in Africa, for example, to a destination in the Mediterranean region. Rather, many birds stop multiple times while migrating to feed and rest, thereby transporting the ticks to each rest stop, depositing them, and picking up new ones that are then deposited farther along the route. While still unproven, this is probably what happens with birds that follow the migratory route along the coast of western Africa, resting every few kilometers and continually dropping and picking new ticks. To support this, empirical evidence indicates that the molecular features of CCHFV found on the western coast of Africa are similar to those of the strain found in southwestern Spain, both points along the main western pathway followed by many migratory birds from Africa to Europe (95).
The spread of CCHFV from its African ancestor to other areas of the world appears to overlap in time with movements of livestock (96). While small- and medium-sized wild mammals serve as hosts for large numbers of immature ticks, domestic and wild ruminants have the potential to feed large numbers of adults (Figure 2). As a result of the substantial tick burdens large vertebrates can support, a large population of infected tick larvae may develop on a single host even if CCHFV transmission efficiency is low. About 500 females may simultaneously feed on a single animal, and each female may lay around 5,000 eggs (1). The data concerning the efficiency of transovarial infection from the female to the egg is unclear, but even with a transovarial transmission efficiency of ~1%, this scenario would generate 25,000 infected larvae from only one host that is not a reservoir, but just the food source for the females. Undoubtedly, the close proximity in the natural habitats of hosts for both immature (i.e., hares) and adult (i.e., ungulates) ticks would result in a self-fueled CCHFV prevalence in ticks. As several studies provide evidence of viremia in livestock, the overlap of feeding adult ticks on infected hosts could further increase the number of infected ticks, but this remains to be demonstrated.
9. Recommendations for future investigations
There are several areas noted throughout this report identifying gaps in knowledge. Here, in summary, we highlight some key questions warranting future investigation.
Experimental design and reporting of data
Key ecological questions were investigated in CCHFV experimental studies by experienced and innovative researchers in the field, but the data that exist have several weaknesses hampering interpretation and comparison. In contrast to seroepidemiological studies, overall, data from experimental infections of wild and domestic animals are limited to only a few groups and relatively few publications. Common limitations in these studies include: small number of animals; variations in inoculum source, passaging, and preparation (Table 4); unclear inoculum dosage based on variations in titration techniques; and confounding by unrelated disease, as was seen in studies by Shepherd et al. (50). These limitations are important to consider in designing future investigations. Transparency in experimental design and data, including reporting of negative data and details of the virus inoculum, are of notable importance in CCHFV studies. Sequencing the isolate and clearly reporting the virus strain, titer techniques, and passage history is critical in future studies. Passaging CCHFV isolates in suckling mice, cell culture, or other means (e.g., ticks, vertebrates) can affect outcome (97), though the relative influences of virus isolation and passage history on experimental outcome are unknown. In addition, early studies were done with virus titrated in NWM. Sensitivity of NWM isolation has not been compared to cell culture isolation. Studies on these fundamental questions are critically lacking.
Table 4. CCHFV strains used in maintenance and transmission experimental infection animal studies.
This table summarizes the variety of isolates that were used in the studies described, including CCHFV strains and passage history. When original isolation information was not reported, year of isolation is listed as ≤ the year in which the earliest published report of the isolate was found.
| Year of isolation | Region | Strain | Source of isolate (reference) | Passage history | Ref. |
|---|---|---|---|---|---|
| 1956 | Congo | UG 3010 | Human (48,102–104) | SMB | (48) |
| 1958 | Uganda | Nakiwogo | Human serum (104) | SMB | (48) |
| 1965 | Nigeria | IbAn 7620 | Goat (48) | 3–4× SMB | (48,77) |
| 1966 | Sokoto, Nigeria | IbAr 10200 | Hyalomma excavatum tick collected from camel (49) | SMB | (48) |
| 1967 | Uzbek SSR | Khodzha (Hodzha) | Human (105) | 29× or 41× SMB | (48) (105) |
| 1968 | Rostov Oblast | Sudarkina | Human (37,44) | 16× SMB | (44) |
| 1968 | Rostov Oblast | Sudarkina | Human (37,44) | 21–48× SMB | (37) |
| 1968 | Rostov Oblast | Sudarkina | Human (37,44) | 42–49× SMB | (46) |
| 1968 | Rostov Oblast | Sudarkina | Human (37,44) | 50× SMB | (35) |
| 1968 | Rostov Oblast | Sudarkina | Human (37,44) | 56–58× SMB | (38) |
| 1968 | Astrakhan Oblast | Hp-16 | Hyalomma marginatum ticks collected from rooks (78) | NR | (78) |
| 1969 | Russia | Hp-15 | Hyalomma marginatum ticks (37) | 6–7× SMB | (37) |
| ≤ 1969 | NR | K67/67 | Human (48,106) | SMB | (48) |
| 1970 | Nigeria | Ar 43665† | Hyalomma rufipes ticks experimentally inoculated with IbAn 7620 (77) | IbAn 7620 (NR), 1× tick | (77) |
| 1976 | Pakistan | Congo, JD 206 | Hyalomma anatolicum ticks (48) | NR | (48) |
| ≤ 1979 | Pakistan | Hazara (JC 280) | Ixodes redikorzevi ticks (48,107) | NR | (48) |
| ≤ 1979 | Nigeria | IbAn 7620† | Atelerix albiventris hedgehog experimentally infected with InAn 7620 (108) | 3× SMB, 1× hedgehog | (108) |
| ≤ 1979 | NR | K128-12 | Hyalomma asiaticum ticks (48) | SMB | (48) |
| 1981 | South Africa | SPU 4/81 | Human, fatal (65) | 2× cell culture, 3× SMB; 2× cell culture, 4× SMB | (36,50,51) |
| 1984 | South Africa | SPU 41/84 | Human, non-fatal (36) | 8× SMB | (36) |
| 1988 | Southern Mauritania | Dak H49199 | Human, fatal (109) | 3× SMB | (35,52,97) |
| 1988 | North-central Senegal | ArD49088 | Hyalomma impeltatum ticks collected from sheep (110) | 3× SMB | (97) |
Abbreviations: NR, not reported; SMB, suckling mouse brain passage or suspension; SSR, Soviet Socialist Republic. Some isolates (denoted with †) were obtained via experimental inoculation of ticks or mammals and re-isolation.
Advanced experimental infection studies of wild vertebrates
Future studies should continue to investigate the roles of wild vertebrate hosts in amplification and transmission of virus, tick-transmission dynamics, and domestic animal infection. Our current knowledge of CCHFV epidemiology has many gaps. The resolution of these gaps needs intensive laboratory work in high-security biosafety conditions, and may be limited by existing transmission model systems (98). Importantly, animal contact has been a direct transmission source of CCHFV to humans. Additional experimental infection studies in species with high risk of infection in nature and frequent human interaction (i.e., livestock and other domestic species) can be used to assess human risk of exposure and disease. Such studies are warranted, and should detail presence or absence of virus in potential sources of infection (e.g., blood, bodily fluids, tissues) by investigating virus dissemination in the host and virus shedding. The importance of viral persistence has recently been reported in cases of other severe hemorrhagic fever viruses (99,100). Investigating viral dissemination and potential persistence in tissues, an area that has never been investigated in animals for CCHFV, is also important for more definitive exclusion of vertebrates as long-term reservoirs of CCHFV.
Investigation of vector-dependent factors in animal studies
Ideally, future studies would mirror natural infection by tick inoculation. However, much work must be done on CCHFV and ticks experimentally to aid study design, and, ultimately, understanding the role of vertebrates in the infection cycle of CCHFV. This work includes determining vector and reservoir competence, including the efficacy of transovarial infection in ticks from infected hosts with detectable viremia, and identifying the contribution of threshold viremia in CCHFV transmission. The ideal conditions for investigating tick transmission dynamics would include experimentally infecting vertebrates identified in CCHFV ecology (e.g., hares), allowing immature ticks to feed on them while viremia is detectable, and then allowing the resulting adult ticks to feed on naive livestock or wild deer. These investigations would require collaboration between virologists and acarologists; resources to maintain tick colonies (e.g., personnel, animals to provide blood meals); access to high-containment facilities able to house a variety of species, including large animals; and the necessary long-term funding for tick studies. Importantly, this multi-faceted experimental approach would provide reliable data on critical aspects of CCHFV maintenance and transmission, including the ability of a putative amplification host to transmit infective CCHFV to feeding immature ticks. In addition to assessing the ability of the infected adult ticks to infect livestock, non-infected ticks can be allowed to feed alongside with the putatively infected female ticks, and CCHFV prevalence can be assessed in the hatching larvae from both sets of ticks. The potential of infection by co-feeding ticks should not be discarded. In any case, the experiments should clarify a pattern regarding the ability of infected animals to infect feeding ticks and to confirm transovarial transmission under controlled conditions.
Considerations for natural tick ecology
Recognizing the central role of ticks in CCHFV transmission and maintenance, experimental infections like those proposed above must be designed with serological and tick ecology data in mind, selecting ticks compatible to hosts that can be infected with CCHFV and are naturally found in the same habitats. Future studies focused on the interplay between hosts and vectors will further our knowledge of viral ecology, and may aid in identifying putative intervention strategies to interrupt natural CCHFV transmission cycles. This work can help refine interventions and identify high-risk scenarios for high-impact epizootics or virus introduction.
This report is one of a series of comprehensive reviews on CCHFV. Further discussion of these and additional unanswered questions on CCHFV ecology and disease will be provided in future reviews on the role of ticks, and on attempts to model human disease in animals.
Highlights final version.
This article is part of a series of reviews of historical data on CCHFV.
Wild and domestic vertebrates infected with CCHFV develop a brief viremia, but do not become ill.
Vertebrates provide blood meals for large numbers of ticks and serve as amplifying hosts.
Viremia is rarely detected in birds, but they may transport CCHFV-infected ticks across wide areas.
Future reviews in the series will focus on attempts to model disease in animals and the role of ticks in virus ecology.
Acknowledgments
The authors would like to thank Alexander Lukashev for critical review and helpful discussion, and Tatyana Klimova for editing the manuscript. The authors would also like to thank and acknowledge Richard Robbins and Terry Carpenter for their efforts to preserve historical Russian-language papers on ticks and tick-borne diseases translated under the direction of Harry Hoogstraal (101).
This work was supported in part by an appointment to the Research Participation Program at CDC by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC (to J.R.S.) and by the National Institutes of Health Loan Repayment Award (to J.R.S.).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15(4):307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- 2.Spengler JR, Bergeron E, Rollin PE. Seroepidemiological Studies of Crimean-Congo Hemorrhagic Fever Virus in Domestic and Wild Animals. Clements ACA, editor. PLoS Negl Trop Dis. 2016;10(1):e0004210. doi: 10.1371/journal.pntd.0004210. [Internet] Available from: http://dx.plos.org/10.1371/journal.pntd.0004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100(1):159–89. doi: 10.1016/j.antiviral.2013.07.006. [Internet] [cited 2014 Oct 10]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/23906741. [DOI] [PubMed] [Google Scholar]
- 4.Mertens M, Schmidt K, Ozkul A, Groschup MH. The impact of Crimean-Congo hemorrhagic fever virus on public health. Antiviral Res. 2013;98(2):248–60. doi: 10.1016/j.antiviral.2013.02.007. [Internet] [cited 2016 Aug 26]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/23458713. [DOI] [PubMed] [Google Scholar]
- 5.Zivcec M, Scholte F, Spiropoulou C, Spengler J, Bergeron E. Molecular Insights into Crimean-Congo Hemorrhagic Fever Virus. Viruses. 2016;8(4):106. doi: 10.3390/v8040106. [Internet] Available from: http://www.mdpi.com/1999-4915/8/4/106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grashchenkov N. Investigations of etiology, pathogenesis, and clinical symptomatology of Crimean hemorrhagic fever (In English: NAMRU3-T1189) Reports 1944 Sci Investig Inst Neurol Akad Med Nauk SSSR; Moskva. 1945:100–7. [Google Scholar]
- 7.Bilibin A. Omsk and Crimean hemorrhagic fevers (In English: NAMRU3-T805) Symptoms Diagnosis Infect Medgiz; Moskva. 1950:200–8. [Google Scholar]
- 8.Neklyudov M. A case of hemorrhagic fever (Crimea) (In English: NAMRU3-T1514) Suvrem Med, Sof. 1952;5:92–5. [Google Scholar]
- 9.Gonzalez JP, Camicas JL, Cornet JP, Faye O, Wilson ML. Sexual and transovarian transmission of Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum ticks. Res Virol. 1992;143(1):23–8. doi: 10.1016/s0923-2516(06)80073-7. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1565850. [DOI] [PubMed] [Google Scholar]
- 10.Honig JE, Osborne JC, Nichol ST. The high genetic variation of viruses of the genus Nairovirus reflects the diversity of their predominant tick hosts. Virology. 2004;318(1):10–6. doi: 10.1016/j.virol.2003.09.021. [Internet] [cited 2013 Apr 8]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/14972529. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn J, Wiley M, Rodriguez S, Bao Y, Prieto K, Travassos da Rosa A, et al. Genomic Characterization of the Genus Nairovirus (Family Bunyaviridae) Viruses. 2016;8(6):164. doi: 10.3390/v8060164. [Internet] Available from: http://www.mdpi.com/1999-4915/8/6/164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahmasebi F, Ghiasi SM, Mostafavi E, Moradi M, Piazak N, Mozafari A, et al. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus genome isolated of ticks from Hamadan province of Iran. J Vector Borne Dis. 2010 Dec;:211–6. [PubMed] [Google Scholar]
- 13.Široky P, Petrželkova KJ, Kamler M, Mihalca AD, Modry D. Hyalomma aegyptium as dominant tick in tortoises of the genus Testudo in Balkan countries, with notes on its host preferences. Exp Appl Acarol. 2006;40(3–4):279–90. doi: 10.1007/s10493-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 14.McCoy KD, Leger E, Dietrich M. Host specialization in ticks and transmission of tickborne diseases: a review. Front Cell Infect Microbiol. 2013;3(October):57. doi: 10.3389/fcimb.2013.00057. [Internet] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3790072&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estrada-Pena A, Jameson L, Medlock J, Vatansever Z, Tishkova F. Unraveling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: a gap analysis for the western Palearctic. Vector Borne Zoonotic Dis. 2012;12(9):743–52. doi: 10.1089/vbz.2011.0767. [Internet] [cited 2013 Apr 8]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22448676. [DOI] [PubMed] [Google Scholar]
- 16.Estrada-Pena A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 2014;108:104–28. doi: 10.1016/j.antiviral.2014.05.016. [Internet] [cited 2014 Oct 9]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24925264. [DOI] [PubMed] [Google Scholar]
- 17.CDC. One Health. [Internet]. [cited 2016 Jun 28]. Available from: https://www.cdc.gov/onehealth/
- 18.Chumakov M, Vafakulov BK, Zavodova T, Karmysheva VY, Maksumov S, Mart’yanova LI, et al. Cases of Crimean hemorrhagic fever transmission in Uzbekistan through contacts with the blood of a sick cow and a patient and also by tickbites (In English: NAMRU3-T1111) In: Chumakov MP, editor. Med Virol Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1974. pp. 29–34. [Google Scholar]
- 19.Nabeth P, Cheikh DO, Lo B, Faye O, Vall IOM, Niang M, et al. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg Infect Dis. 2004;10(12):2143–9. doi: 10.3201/eid1012.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mostafavi E, Chinikar S, Moradi M, Bayat N, Meshkat M, Fard MK, et al. A case report of Crimean Congo hemorrhagic fever in ostriches in Iran. Open Virol J. 2013;7:81–3. doi: 10.2174/1874357901307010081. [Internet] [cited 2014 Oct 10] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3763622&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Work TH. Russian spring-summer virus in India: Kyasanur Forest disease. Prog Med Virol. 1958;1:248–279. [Internet] Available from: http://www-ncbi-nlm-nih-gov.proxy2.lib.umanitoba.ca/pubmed/13579010. [PubMed] [Google Scholar]
- 22.Softly A, Stanley NF. The use of sentinel infant mice for virus isolation. Aust J Exp Biol Med Sci. 1965 Apr;43:171–4. doi: 10.1038/icb.1965.15. [DOI] [PubMed] [Google Scholar]
- 23.Khodukin N. Viral diseases in Uzbek SSR (In English: NAMRU3-T1292) Vestn Akad Med Nauk SSSR. 1948;2:50–2. [Google Scholar]
- 24.Belyaeva A, Karmysheva VY, Chumakov MP. Application of the fluorescent antibody method (FAT) for identifying Crimean hemorrhagic fever (CHF) virus in pig embryo kidney (PEK) cell culture (In English: NAMRU3-T795) Mater 11 Nauch Sess Inst Polio Virus Entsef (Moscow, 1964) 1964:295. [Google Scholar]
- 25.Chumakov M, Shalunova N, Semashko I, Belyaeva A. Use of interference phenomenon in tissue culture for detecting Crimean hemorrhagic fever virus (CHF) (In English: NAMRU3-T832) In: Chumakov MP, editor. Viral Hemorrhagic Fevers Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1965. pp. 202–208. [Google Scholar]
- 26.Chumakov M, Belyaeva A, Voroshilova M, Butenko A, Shalunova N, Semashko I, et al. Progress in studying the etiology, immunology, and laboratory diagnosis of Crimean hemorrhagic fever in the USSR and Bulgaria (In English: NAMRU3-T613). Tickborne Enceph hemorrhagic fevers, mosquitoborne arboviral Infect Ed by Chumakov, MP Mater 15 Nauch Sess Inst Polio Virus Entsef; Moscow. Oct 21–25, 1968; 1968. pp. 100–103. [Google Scholar]
- 27.Chumakov M. Etiology and epidemiology of hemorrhagic fevers (In English: NAMRU3-T1035) Ter Arkv. 1948;2:85–86. [Google Scholar]
- 28.Butenko A, Chumakov M, Bashkirtsev V, Zavodova T, Tkachenko E, Rubin S, et al. Isolation and characterization of Astrakhan strain (Drozdov) of Crimean hemorrhagic fever virus and data on serodiagnosis of this infection (In English: NAMRU3-T866) Mater 15 Nauch Sess Inst Polio Virus Entsef. 1968;3:88–90. [Google Scholar]
- 29.Karmysheva VY, Borisov V, Tkachenko E, Butenko A, Chumakov M. Characteristics of certain cell cultures infected with rodent-pathogenic Crimean hemorrhagic fever (CHF) virus strain (In English: NAMRU3-T867) Mater 15 Nauch Sess Inst Polio Virus Entsef. 1968;3:92–4. [Google Scholar]
- 30.Chumakov M. Contribution to 30 years of investigation of Crimean hemorrhagic fever (In English: NAMRU3-T950) Med Virol Ed by Chumakov, MP Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1974;22(2):5–18. [Google Scholar]
- 31.Hoch T, Breton E, Josse M, Deniz A, Guven E, Vatansever Z. Exp Appl Acarol. 3. Vol. 68. Springer International Publishing; 2016. Identifying main drivers and testing control strategies for CCHFV spread; pp. 347–59. [Internet] Available from: “ http://dx.doi.org/10.1007/s10493-015-9937-9. [DOI] [PubMed] [Google Scholar]
- 32.Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6(4):203–14. doi: 10.1016/S1473-3099(06)70435-2. [Internet] [cited 2013 Apr 8]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/16554245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birulya N, Zalutskaya L, Perelatov V. Distribution area of natural foci of Crimean hemorrhagic fever (In English: NAMRU3-T962) Viral hemorrhagic fevers Crime hemorrhagic fever, Omsk hemorrhagic fever, hemorrhagic fever with Ren Syndr Chumakov, MP Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1971;19:180–5. [Google Scholar]
- 34.Zgurskaya GN, Berezin V, Smirnova S, Chumakov M. Investigation of the question of Crimean hemorrhagic fever virus transmission and interepidemic survival in the tick Hyalomma plumbeum plumbeum Panzer (In English: NAMRU3-T911) In: Chumakov M, editor. Viral hemorrhagic fevers Crime hemorrhagic fever, Omsk hemorrhagic fever, hemorrhagic fever with Ren syndrone Tr Inst Polio Virus Entsef Akad Med Nauk SSSP. Vol. 19. 1971. pp. 217–20. [Google Scholar]
- 35.Perelatov V, Kuchin V, Donets M, Zarubina L, Kondratenko VF, Blagoveshchenskaya NM, Vostokova K, Novikova L, et al. Results on experimental infection of European hares with Crimean hemorrhagic fever virus (In English: NAMRU3-T1032). Tezisy 17 Nauch Sess Inst Posvyashch Aktual Probl Virus Profil Virus Zabolev; Moscow. October; 1972; pp. 354–5. [Google Scholar]
- 36.Shepherd AJ, Leman PA, Swanepoel R. Viremia and antibody response of small African and laboratory animals to Crimean-Congo hemorrhagic fever virus infection. Am J Trop Med Hyg. 1989;40(5):541–7. doi: 10.4269/ajtmh.1989.40.541. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2499205. [DOI] [PubMed] [Google Scholar]
- 37.Blagoveshchenskaya NM, Vyshnivetskaya L, Gusarev A, Zarubina L, Kondratenko VF, Kuchin V, et al. Investigation of susceptibility of little susliks (Citellus pygmaeus) to CHF virus (In English: NAMRU3-T1064). In: Chumakov MP, editor. Actual Probl Virol Prophyl viral Dis; Tezisy 17 Nauch Sess Inst Posvyashch Aktual Probl Virus Profil Virus Zabolev; Moscow. Oct 1972; 1972. p. 356. [Google Scholar]
- 38.Blagoveshchenskaya N, Donets M, Zaruna L, Kondratenko VF, Kuchin V. Tezisy Konf Vop Med Virus (Moscow, Oct 1975) 1975. Study of susceptibility to Crimean hemorrhagic fever (CHF) virus in European and long-eared hedgehogs (In English: NAMRU3-T985) pp. 269–70. [Google Scholar]
- 39.Mustafa ML, Ayazi E, Mohareb E, Yingst S, Zayed A, Rossi C, et al. Crimean-Congo haemorrhagic fever, Afghanistan, 2009. Emerg Infect Dis. 2011;17(10):1940–1. doi: 10.3201/eid1710.110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chinikar S, Ghiasi SM, Moradi M, Goya MM, Shirzadi MR, Zeinali M, et al. Geographical distribution and surveillance of Crimean-Congo hemorrhagic fever in Iran. Vector Borne Zoonotic Dis. 2010;10(7):705–8. doi: 10.1089/vbz.2009.0247. [Internet] [cited 2013 Apr 8]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/20854025. [DOI] [PubMed] [Google Scholar]
- 41.Sharifi-Mood B, Metanat M, Alavi-Naini R. Prevalence of Crimean-Congo hemorrhagic fever among high risk human groups. Int J high risk Behav Addict. 2014;3(1):e11520. doi: 10.5812/ijhrba.11520. [Internet] [cited 2014 Oct 10] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4070186&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadanali A, Erol S, Ozkurt Z, Ozden K. Epidemiological risk factors for Crimean-Congo hemorrhagic fever patients. Turk J Med Sci. 2009;39(13):829–32. [Google Scholar]
- 43.Sargianou M, Panos G, Tsatsaris A, Gogos C, Papa A. Int J Infect Dis. 12. Vol. 17. International Society for Infectious Diseases; 2013. Crimean-Congo hemorrhagic fever: seroprevalence and risk factors among humans in Achaia, western Greece; pp. e1160–5. [Internet] [cited 2014 Oct 10] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24084247. [DOI] [PubMed] [Google Scholar]
- 44.Milyutin V, Butenko A, Artyushenko A, Bliznichenko A, Zavodova T, Zarubina LV, et al. Experimental infection of horses with Crimean hemorrhagic fever virus. Report 1: Clinical observations (In English: NAMRU3-T851). Arboviruses, Ed by Chumakov, MP Mater 16 Nauch Sess Inst Polio Virus Entsef; Moscow. Oct 21–23, 1969; Moscow: Oct, 1969. pp. 145–6. [Google Scholar]
- 45.Gonzalez J, Camicas J, Cornet J, Wilson M. Biological and clinical responses of West African sheep to Crimean-Congo haemorrhagic fever virus experimental infection. Res Virol. 1998;149(6):44. doi: 10.1016/s0923-2516(99)80013-2. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9923021. [DOI] [PubMed] [Google Scholar]
- 46.Zarubinsky VY, Kondratenko VF, Blagoveshchenskaya NM, Zarubina LV, Kuchin VV. Susceptibility of calves and lambs to Crimean hemorrhagic fever virus (In English: NAMRU3-T1178). Tezisy Dokl 9 Vses Konf Prir Ochag Bolez Chelov Zhivot; Omsk. May 1976; Omsk: 1976. pp. 130–1. [Google Scholar]
- 47.Gaidamovich S, Klisenko G, Shanoyan N, Obukhova V, Melnikova E. Indirect hemagglutination for diagnosis of Crimean hemorrhagic fever. Intervirology. 1974;2:181–5. doi: 10.1159/000149421. [DOI] [PubMed] [Google Scholar]
- 48.Smirnova SE. A Comparative Study of the Crimean Hemorrhagic Fever-Congo Group of Viruses. Arch Virol. 1979;62(2):137–43. doi: 10.1007/BF01318066. [DOI] [PubMed] [Google Scholar]
- 49.Causey OR, Kemp GE, Madbouly MH, David-West TS. Congo virus from domestic livestock, African hedgehog, and arthropods in Nigeria. Am J Trop Med Hyg. 1970;19(5):846–50. doi: 10.4269/ajtmh.1970.19.846. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd AJ, Swanepoel R, Shepherd SP, Leman PA, Mathee O. Viraemic transmission of Crimean-Congo haemorrhagic fever virus to ticks. Epidemiol Infect. 1991;106(2):373–82. doi: 10.1017/s0950268800048524. [Internet] [cited 2014 Oct 10]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2272004&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepherd AJ, Swanepoel R, Cornel A, Mathee O. Experimental studies on the replication and transmission of Crimean-Congo hemorrhagic fever virus in some African tick species. Am J Trop Med Hyg. 1989;40(3):326–31. doi: 10.4269/ajtmh.1989.40.326. [DOI] [PubMed] [Google Scholar]
- 52.Wilson ML, Gonzalez JP, Cornet JP, Camicas JL. Transmission of Crimean-Congo haemorrhagic fever virus from experimentally infected sheep to Hyalomma truncatum ticks. Res Virol. 1991;142(5):395–404. doi: 10.1016/0923-2516(91)90007-p. [Internet] [cited 2014 Oct 10]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/1771294. [DOI] [PubMed] [Google Scholar]
- 53.Rabinovich V, Milyutin V, Artyushenko A, Buryakov B, Chumakov M. Possibility of extracting hyperimmune gammaglobulin against CHF from donkey blood sera (In English: NAMRU3-T1059). In: Chumakov MP, editor. Actual Probl Virol Prophyl viral Dis; Tezisy 17 Nauch Sess Inst Posvyashch Aktual Probl Virus Profil Virus Zabolev; Moscow. Oct 1972; Moscow: Oct, 1972. pp. 350–1. [Google Scholar]
- 54.Grobov A. Carriers of Crimean haemorrhagic fever (In English: NAMRU3-T36) Med Parazitol Parazit Bolezn. 1946;15:59–63. [Google Scholar]
- 55.Berezin VV, Povalishina TP, Ermakova RM, Stolbov DN. On the role of birds in feeding immature stages of Hyalomma plumbeum plumbeum ticks-vectors of hemorrhagic fever of the Crimean type in foci of the Volga Delta (In English: NAMRU3-Tl98) Sborn Tr Inst Polio Virus, Encef Akad Med Nauk USSR, (Medicine, Moscow) 1965;7:296–303. [Google Scholar]
- 56.Zimina YV, Birulya N, Berezin V, Zalutskaya L, Povalishina TP, Stolbov D. Sborn Tr Inst Polio Virus, Encef Akad Med Nauk USSR, (Medicine, Moscow) 1965. Materials on zoologico-parasitologic characteristics of Crimean hemorrhagic fever in Astrakhan Oblast (In English: NAMRU3-T197) pp. 288–95. [Google Scholar]
- 57.Chumakov M, Zavodova T, Mart’yanova LI, Mukhirtdinov A, Povalishchina T, Rodin V, et al. Detection of Crimean hemorrhagic fever in a few bloodsucking ticks species collected in 1973 in Kirgiz SSR and Uzbek SSR (In English: NAMRU3-T1112) Med Virol Ed by Chumakov, MP Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1974;22(2):35–39. [Google Scholar]
- 58.Zeller HG, Cornet JP, Camicas JL. Crimean-Congo haemorrhagic fever virus infection in birds: field investigations in Senegal. Res Virol. 1994;145(2):105–9. doi: 10.1016/s0923-2516(07)80012-4. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/8059064. [DOI] [PubMed] [Google Scholar]
- 59.Labuda M, Danielova V, Jones LD, Nuttall PA. Amplification of tick-borne encephalitis virus infection during co-feeding of ticks. Med Vet Entomol. 1993;7(4):339–42. doi: 10.1111/j.1365-2915.1993.tb00702.x. [Internet] [cited 2016 Apr 4]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/8268488. [DOI] [PubMed] [Google Scholar]
- 60.Logan TM, Linthicum KJ, Bailey CL, Watts DM, Moulton JR. Experimental Transmission of Crimean-Congo Hemorrhagic Fever Virus by Hyalomma Truncatum Koch. Am J Trop Med Hyg. 1989;40(2):207–12. doi: 10.4269/ajtmh.1989.40.207. [DOI] [PubMed] [Google Scholar]
- 61.Berezin V, Chumakov M, Rubin S, Stolbov D, Butenko A, Bashkirtsev V. hContribution to the ecology of Crimean hemorrhagic fever virus in the lower Volga River (In English: NAMRU3-T836). Arboviruses, Ed by Chumakov, MP Mater 16 Nauch Sess Inst Polio Virus Entsef; Moscow. Oct 21–23, 1969; 1969. pp. 120–2. [Google Scholar]
- 62.Chunikhin SP. Investigation of the ecology of arboviruses in arid regions of Central Asia and Africa (In English: NAMRU3-1098). In: Chumakov M, Chumakov MP, editors. Actual Probl Virol Prophyl viral Dis; Tezisy 17 Nauch Sess Inst Posvyashch Aktual Probl Virus Profil Virus Zabolev; Moscow. Oct 1972; Moscow: Oct, 1972. pp. 272–275. [Google Scholar]
- 63.Berezin V, Chumakov M, Reshetnikov I, Zgurskaya G. Study of the role of birds in the ecology of Crimean hemorrhagic fever virus. 1971:94–5. [Google Scholar]
- 64.Shepherd A, Swanepoel R, Leman P, Shepherd SP. Field and laboratory investigation of Crimean-Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Trans R Soc Trop Med Hyg. 1987;81:1004–7. doi: 10.1016/0035-9203(87)90379-8. [DOI] [PubMed] [Google Scholar]
- 65.Swanepoel R, Struthers J, Shepherd A, McGillivray G, Nel N, Jupp P. Crimean-Congo Hemorrhagic Fever in South Africa. Am J Trop Med Hyg. 1983;32(6):1407–15. doi: 10.4269/ajtmh.1983.32.1407. [DOI] [PubMed] [Google Scholar]
- 66.Shepherd AJ, Swanepoel R, Leman PA. Antibody Response in Crimean-Congo Hemorrhagic Fever. Rev Infect Dis. 1989;11:S801–6. doi: 10.1093/clinids/11.supplement_4.s801. [DOI] [PubMed] [Google Scholar]
- 67.Zeller HG, Cornet JP, Camicas JL. Experimental transmission of Crimean-Congo hemorrhagic fever virus by west African wild ground-feeding birds to Hyalomma marginatum rufipes ticks. Am J Trop Med. 1994;50(6):676–81. doi: 10.4269/ajtmh.1994.50.676. [DOI] [PubMed] [Google Scholar]
- 68.Xia H, Zhao J, Li Y, Yin S, Tang S, Zhang Z, et al. Virus Res. Vol. 173. Elsevier B.V: 2013. Infection and propagation of Crimean-Congo hemorrhagic fever virus in embryonated chicken eggs; pp. 344–9. [Internet] [cited 2013 Apr 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23352881. [DOI] [PubMed] [Google Scholar]
- 69.Swanepoel R, Leman PA, Burt FJ, Jardine J, Verwoerd DJ, Capua I, et al. Experimental infection of ostriches with Crimean-Congo haemorrhagic fever virus. Epidemiol Infect. 1998;121(2):427–32. doi: 10.1017/s0950268898001344. [Internet] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2809542&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chumakov M. A new virus disease - Crimean hemorrhagic fever (Russian) Nov Med. 1947;4:9–11. [Google Scholar]
- 71.Chumakov M. Encycl Mil Med. Vol. 3. Moscow: 1948. Crimean Hemorrhagic Fever. [Google Scholar]
- 72.Gonzalez JP, Cornet JP, Wilson ML, Camicas JL. Crimean-Congo haemorrhagic fever virus replication in adult Hyalomma truncatum and Amblyomma variegatum ticks. Res Virol. 1991;142(6):483–8. doi: 10.1016/0923-2516(91)90071-a. [Internet] [cited 2014 Oct 10]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/1803413. [DOI] [PubMed] [Google Scholar]
- 73.Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci (Landmark Ed. 2009;14:2051–88. doi: 10.2741/3363. [Internet] [cited 2016 Feb 25] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2785505&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nuttall PA, Labuda M. Tick-host interactions: saliva-activated transmission. Parasitology. 2004;129(Suppl):S177–89. doi: 10.1017/s0031182004005633. [Internet] [cited 2016 Apr 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15938511. [DOI] [PubMed] [Google Scholar]
- 75.Titus RG, Ribeiro JM. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today. 1990;6(5):157–60. doi: 10.1016/0169-4758(90)90338-5. [Internet] [cited 2016 Feb 18]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/15463328. [DOI] [PubMed] [Google Scholar]
- 76.Gordon SW, Linthicum KJ, Moulton JR. Transmission of Crimean-Congo hemorrhagic fever virus in two species of Hyalomma ticks from infected adults to cofeeding immature forms. Am J Trop Med Hyg. 1993;48(4):576–80. doi: 10.4269/ajtmh.1993.48.576. [DOI] [PubMed] [Google Scholar]
- 77.Lee V, Kemp G. Congo virus: experimental infection of Hyalomma rufipes and transmission to a calf. Bull Ent Soc Niger. 1970;2:133–5. [Google Scholar]
- 78.Berezin V. Investigation of the ecology of arboviruses in river deltas of the Caspian and Azov Sea basins (In English: NAMRU3-T1160) Avtoref Diss Soisk Uchen Step Dokt Biol Nauk Inst Polio Virus Entsef Akad Med Nauk SSSR. 1971:37. [Google Scholar]
- 79.Zgurskaya GN. Threshold levels of blood infectiousness for Hyalomma p. plumbeum tick during viremia caused by CHF virus in hares and rabbits (In English: NAMRU3-T997) Tezisy Konf Vop Med Virus (Moscow, Oct 1975) 1975:291–2. [Google Scholar]
- 80.Levi V, Vasilenko S. Study of the Crimean hemorrhagic fever (CHF) virus transmission in mechanism in Hyalomma p. plumbeum ticks (In English: NAMRU3-T982) Epidem Mikrobiol Infekts Bol. 1978;9(2):182–5. [Google Scholar]
- 81.Kondratenko VF. Importance of Ixodid ticks in transmitting and preserving the Crimean hemorrhagic fever agent in infection foci (In English: NAMRU3-T1116) Parazitol Leningr. 1976;10(4):297–302. [PubMed] [Google Scholar]
- 82.Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip Perspect Infect Dis. 2009:593232. doi: 10.1155/2009/593232. [Internet] [cited 2016 Aug 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19277106. [DOI] [PMC free article] [PubMed]
- 83.Estrada-Pena A, Martinez Aviles M, Munoz Reoyo MJ. A population model to describe the distribution and seasonal dynamics of the tick Hyalomma marginatum in the Mediterranean Basin. Transbound Emerg Dis. 2011;58(3):213–23. doi: 10.1111/j.1865-1682.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 84.Leblebicioglu H, Sunbul M, Memish ZA, Al-Tawfiq JA, Bodur H, Ozkul A, et al. Int J Infect Dis. Vol. 38. International Society for Infectious Diseases; 2015. Consensus report: Preventive measures for Crimean-Congo Hemorrhagic Fever during Eid-al-Adha festival; pp. 9–15. [Internet] Available from: http://www.sciencedirect.com/science/article/pii/S1201971215001678. [DOI] [PubMed] [Google Scholar]
- 85.Mohd Shukri M, Ling Kho K, Ghane Kisomi M, Lani R, Marlina S, Muhd Radzi SF, et al. BMC Public Health. Vol. 15. BMC Public Health; 2015. Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian’s farm workers; p. 704. [Internet] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4513429&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nuttall PA, Trimnell AR, Kazimirova M, Labuda M. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 2006;28(4):155–163. doi: 10.1111/j.1365-3024.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 87.Labuda M, Trimnell AR, Ličkova M, Kazimirova M, Davies GM, Lissina O, et al. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006;2(4):251–259. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merino O, Alberdi P, Perez de la Lastra JM, de la Fuente J. Tick vaccines and the control of tick-borne pathogens. Front Cell Infect Microbiol. 2013 Jul;3:30. doi: 10.3389/fcimb.2013.00030. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23847771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buttigieg KR, Dowall SD, Findlay-Wilson S, Miloszewska A, Rayner E, Hewson R, et al. A novel vaccine against Crimean-Congo Haemorrhagic Fever protects 100% of animals against lethal challenge in a mouse model. PLoS One. 2014;9(3):e91516. doi: 10.1371/journal.pone.0091516. [Internet] [cited 2015 Jan 15] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3951450&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dowall SD, Buttigieg KR, Findlay-Wilson SJD, Rayner E, Pearson G, Miloszewska A, et al. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum Vaccines Immunother. 2016;12(2):519–27. doi: 10.1080/21645515.2015.1078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labuda M, Jones LD, Williams T, Danielova V, Nuttall PA. Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J Med Entomol. 1993;30(1):295–9. doi: 10.1093/jmedent/30.1.295. [Internet] [cited 2016 Aug 26]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/8433342. [DOI] [PubMed] [Google Scholar]
- 92.Jones LD, Davies CR, Steele GM, Nuttall PA. A novel mode of arbovirus transmission involving a nonviremic host. Science. 1987;237(4816):775–7. doi: 10.1126/science.3616608. [DOI] [PubMed] [Google Scholar]
- 93.Jones LD, Hodgson E, Nuttall PA. Enhancement of virus transmission by tick salivary glands. J Gen Virol. 1989;70(1989):1895–8. doi: 10.1099/0022-1317-70-7-1895. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2544668. [DOI] [PubMed] [Google Scholar]
- 94.Hasle G, Bjune G, Edvardsen E, Jakobsen C, Linnehol B, Roer JE, et al. Transport of ticks by migratory passerine birds to Norway. J Parasitol. 2009;95(6):1342–51. doi: 10.1645/GE-2146.1. [Internet] [cited 2016 Apr 25]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/19658452. [DOI] [PubMed] [Google Scholar]
- 95.Estrada-Pena A, Palomar A, Santibanez P, Sanchez N, Habela M, Portillo A, et al. Crimean-Congo Hemorrhagic Fever Virus in Ticks, Southwestern Europe, 2010. Emerg Infect Dis. 2012;18(1):179–80. doi: 10.3201/eid1801.111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carroll SA, Bird BH, Rollin PE, Nichol ST. Mol Phylogenet Evol. 3. Vol. 55. Elsevier Inc: 2010. Ancient common ancestry of Crimean-Congo hemorrhagic fever virus; pp. 1103–10. [Internet] [cited 2013 Apr 8]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/20074652. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez JP, Wilson ML, Cornet JP, Camicas JL. Host-passage-induced phenotypic changes in crimean-congo haemorrhagic fever virus. Res Virol. 1995;146(2):131–40. doi: 10.1016/0923-2516(96)81082-x. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/7638437. [DOI] [PubMed] [Google Scholar]
- 98.Gargili A, Thangamani S, Bente D. Influence of laboratory animal hosts on the life cycle of Hyalomma marginatum and implications for an in vivo transmission model for Crimean-Congo hemorrhagic fever virus. Front Cell Infect Microbiol. 2013;3:39. doi: 10.3389/fcimb.2013.00039. [Internet] [cited 2014 Oct 10] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3747357&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med. 2015;372(25):2423–2427. doi: 10.1056/NEJMoa1500306. [Internet] Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deen GF, Knust B, Broutet N, Sesay FR, Formenty P, Ross C, et al. Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors — Preliminary Report. N Engl J Med. 2015 doi: 10.1056/NEJMoa1511410. [Internet] Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1511410. [DOI] [PMC free article] [PubMed]
- 101.Robbins RG, Lazukina I, Apanaskevich DA, Carpenter TL. An annotated list of source publication citations for Russian-language papers on ticks and tick-borne diseases translated under the direction of Harry Hoogstraal, ca. 1967 – 1986. Syst Appl Acarol. 2014;19(1):1–43. [Internet] Available from: http://dx.doi.org/10.11158/saa.19.1.1. [Google Scholar]
- 102.Ahmed AA, McFalls JM, Hoffmann C, Filone CM, Stewart SM, Paragas J, et al. Presence of broadly reactive and group-specific neutralizing epitopes on newly described isolates of Crimean-Congo hemorrhagic fever virus. J Gen Virol. 2005;86(Pt 12):3327–36. doi: 10.1099/vir.0.81175-0. [Internet] [cited 2013 Apr 8] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16298978. [DOI] [PubMed] [Google Scholar]
- 103.Simpson DI, Knight EM, Courtois G, Williams MC, Weinbren MP, Kibukamusoke JW. Congo virus: a hitherto undescribed virus occurring in Africa. I. Human isolations--clinical notes. East Afr Med J. 1967;44(2):86–92. [Internet] [cited 2016 Jul 5]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/6040759. [PubMed] [Google Scholar]
- 104.Woodall JP, Williams MC, Simpson DI. Congo virus: a hitherto undescribed virus occurring in Africa. II. Identification studies. East Afr Med J. 1967;44(2):93–8. [Internet] [cited 2016 Jul 7]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/6068614. [PubMed] [Google Scholar]
- 105.Smirnova SE, Shalunova NV, Mart’yanova LI. Investigation of Samarkhand and Rostov strains of Crimean hemorrhagic fever virus (In English: NAMRU3-T868). Tickborne Enceph hemorrhagic fevers, mosquitoborne arboviral Infect Ed by Chumakov, MP Mater 15 Nauch Sess Inst Polio Virus Entsef; Moscow. Oct 21–25, 1968; 1968. pp. 96–7. [Google Scholar]
- 106.Donets MA, Chumakov MP, Korolev MB, Rubin SG. Physicochemical Characteristicsdon, Morphology and Morphogenesis of Virions of the Causative Agent of Crimean Hemorrhagic Fever. Intervirology. 1977;8:294–308. doi: 10.1159/000148904. [DOI] [PubMed] [Google Scholar]
- 107.Smirnova SE, Shestopalova NM, Reingold VN, Zubri GL, Chumakov MP. Experimental Hazara Virus infection in mice. Acta Virol. 1977;21(2):128–32. [Internet] [cited 2016 Jul 7]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/17280. [PubMed] [Google Scholar]
- 108.Fagbami AH, Tomori O, Fabiyi A, Isoun T. Experimental Congo virus (Ib-AN 7620) infection in primates. Virologie. 1975;26(1):33–7. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/814708. [PubMed] [Google Scholar]
- 109.Gonzalez JP, LeGuenno B, Guillaud M, Wilson ML. A fatal case of Crimean-Congo haemorrhagic fever in Mauritania: virological and serological evidence suggesting epidemic transmission. Trans R Soc Trop Med Hyg. 1990;84(4):573–6. doi: 10.1016/0035-9203(90)90045-g. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez JP, Guillaud M, Wilson ML. Laboratoire d’Ecologie Virale. In: Digoutte JP, editor. Rapp sur le Fonct Tech l’Institut Pasteur Dakar. 1988. p. 106. [Google Scholar]