Abstract
This study aimed at exploring the psychometric characteristics of the Korean Version of the Depression and Somatic Symptoms Scale (DSSS) in a clinical sample, and investigating the impact of somatic symptoms on the severity of depression. Participants were 203 consecutive outpatients with current major depressive disorders (MDD) or lifetime diagnosis of MDD. The DSSS was compared with the Montgomery-Åsberg Depression Rating Scale (MADRS) and the 17-items Hamilton Depression Rating Scale (HAMD). The DSSS showed a two-factor structure that accounted for 56.8% of the variance, as well as excellent internal consistency (Cronbach’s alpha = 0.95), concurrent validity (r = 0.44–0.82), and temporal stability (intraclass correlation coefficient = 0.79). The DSSS had a high ability to identify patients in non-remission (area under receiver operating characteristic [ROC] curve = 0.887). Maximal discrimination between remission and non-full remission was obtained at a cut-off score of 22 (sensitivity = 82.1%, specificity = 81.4%). The number of somatic symptoms (the range of somatic symptoms) and the scores on the somatic subscale (SS, the severity of somatic symptoms) in non-remission patients were greater than those in remission patients. The number of somatic symptoms (slope = 0.148) and the SS score (slope = 0.472) were confirmed as excellent predictors of the depression severity as indicated by the MADRS scores. The findings indicate that the DSSS is a useful tool for simultaneously, rapidly, and accurately measuring depression and somatic symptoms in clinical practice settings and in consultation fields.
Keywords: Depression and Somatic Symptoms Scale, Depression, Somatic Symptoms, Psychometrics, Validation, Cut-off Score
Graphical Abstract
INTRODUCTION
Somatic symptoms, including pain, are a common feature in patients with depression (1,2). The negative effects of somatic symptoms on the diagnosis, treatment, and prognosis of depression are as follows: 1) they mask psychological information regarding depression, making it difficult to confirm the diagnosis of depression (3), 2) they prolong the duration of depressive episodes and increase the severity of depression (4), and 3) they often remain as residual symptoms even after the depression has been appropriately treated, thereby resulting in a decrease in the remission rate of depression and an increase in the recurrence rate of depression (5).
Therefore, somatic symptoms should be frequently measured in patients with depression. In an actual clinical practice, however, clinicians often preferentially concentrate their efforts on making a diagnosis and measuring the severity of the depression, due to the limitations of human resources, time, and costs. As a result, information regarding somatic symptoms is simply obtained from physical and medical histories, and the evaluation of somatic symptoms is frequently delayed (6).
Typical depression scales are thought not to sufficiently include items associated with somatic symptoms (7). Thus, it can be challenging to monitor the somatic dimension of depression during treatment. In addition, it is difficult to accurately measure chronic, persistent somatic symptoms that remain as residual symptoms after appropriate treatment. A separate instrument to measure somatic symptoms can be added to typical depression scales, such as the Symptom Check List-90 (SCL-90) (8) and the Patient Health Questionnaire (PHQ-15) (9). However, it may be challenging to use such scales for every depressed patient in a busy setting of clinical practice. Moreover, it is uncertain whether these somatic scales can accurately reflect the somatic severity of depression in patients because they are not specifically designed for depression.
Thus, depression and somatic symptoms should be measured together via brief, validated self-report questionnaires. If a combined score of depression and somatic symptoms were prepared, clinicians and researchers would be freed from considering the complicated interactions between the two symptom groups (10).
The Depression and Somatic Symptoms Scale (DSSS), which is a self-report questionnaire, was developed with abovementioned background (11). It is composed of 22 items, with 2 major subscales: a depression subscale (DS) and a somatic subscale (SS). The DS has 12 items, and the SS has 10 items. On the SS, 5 items compose the pain subscale (PS). The severity of the symptoms experienced in the last week is scored for each item on a scale from 0 to 3 (0 = absent, 1 = mild, 2 = moderate, 3 = severe). The total scores range from 0 to 66 for the DSSS, 0 to 36 for the DS, and 0 to 30 for the SS. The DS items were prepared to reflect the DSM-IV criteria for the major depressive disorder (MDD), as well as the depression items of the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating Scale (HAMD). The SS items were prepared to reflect the somatic items of the HAMD and SCL-90 scales, as well as the somatic symptoms considered common in depression in previous studies. The SS items were selected based on the following criteria: 1) they reflect the severity of depression and can predict the occurrence of depression, and 2) they have a significant impact on clinical practice and quality of life. The SS items are those that are most critical and close to depression in order to improve the Cronbach’s alpha value of the DSSS. In previous studies (10,11), Cronbach’s alpha values of the DSSS and its subscales ranged from 0.73 to 0.94. Pearson correlation coefficients between DSSS and HAMD ranged from 0.63 to 0.86. The DS and SS significantly correlated with mental and physical dimensions of the Short Form 36, respectively. To our knowledge, this is the first validation of translated version of this instrument. Since the DSSS was introduced, many researchers are interested in this measure, because the DSSS may overcome the deficiency of other depression scale with few somatic items.
The present study aimed to explore the psychometric characteristics of the Korean version of the DSSS in a clinical outpatient sample, addressing the following measurement properties: internal consistency, concurrent validity, temporal stability, factorial validity, optimal cut-off scores in identifying non-full remission. In addition, we investigated the impact of somatic symptoms on the severity of depression.
MATERIALS AND METHODS
Participants
We conducted a prospective, observational, single-site (Korea University Ansan Hospital, Ansan, Korea) study with follow-up in a typical clinical setting of outpatient psychiatric facilities. Study participants were recruited from consecutive outpatients, 18–65 years of age, who met the DSM-IV-TR diagnostic criteria for major depressive disorder (current MDD) or who had suffered from major depressive disorder (lifetime diagnosis of MDD). All participants were assessed under their treatment as usual, which could be long-term ongoing or recently prescribed.
To minimize confounding of somatic symptoms, the following exclusion criteria were established: 1) history of substance dependence or abuse, 2) psychotic symptoms, catatonic features, or severe psychomotor retardation, 3) pregnant or breastfeeding patients, and 4) medical or surgical disorders that had developed in the month before the recruitment.
Procedure
The Korean version of the DSSS was developed using the forward-backward translation process. In the translation process, the items were first translated into Korean by a psychiatrist whose native language was Korean. Two other bilingual Korean professionals performed the back translation blindly. Next, the English back-translated items were compared with the original. If a back-translated item did not agree with the original, revision and back translation were performed repeatedly until the items were consistent with the intent of the original instrument.
After the subjects were recruited, they completed the DSSS and psychometric instruments including a questionnaire on socio-demographic factors. A psychiatrist evaluated the MADRS (12) and the 17-items HAMD (13), which have been validated in Korea (14,15). Ratings on the MADRS and the HAMD were blind to the responses given by patients on the self-reported measures. To examine the temporal stability of the DSSS, patients were required to complete the DSSS a second time 4 weeks after baseline measurement.
The Montgomery-Åsberg Depression Rating Scale (MADRS)
The MADRS is a 10-item depression rating scale, widely used in depressed patients (12). Each item is rated from 0 to 6, allowing a maximum score of 60. The MADRS was designed to measure the severity of depression in clinical samples and to be sensitive to change during antidepressant treatment. The Cronbach’s alpha coefficient of the Korean version of the MADRS was 0.79 (14). The MADRS was used to examine the construct validity of the DS in the DSSS.
The 17-items Hamilton Depression Rating Scale (HAMD)
The HAMD as used in this study is a 17-item clinician-administered measure of depressive symptomatology (13). Items on the HAMD-17 consist of groups of graded statements reflecting increasing severity of symptoms of depression. The Cronbach’s alpha coefficient of the Korean version of the HAMD-17 was 0.88 (15). Full remission of a major depressive episode was defined as a HAMD-17 score ≤ 7, which is the most frequently cited definition of remission (16).
Five items from the HAMD-17 that evaluate somatic symptoms were identified and extracted: anxiety-somatic, somatic symptoms-GI, somatic symptoms-general, hypochondriasis, and loss of weight. This group of items formed a subscale subsequently referred to as the HAMD-S with maximum total score of 14. The HAMD-S was used to examine the construct validity of the SS in the DSSS.
Data analyses
Missing data were replaced with the median of the complete data for each item. The number of missing values for each item was less than 1.0%. To evaluate the structure of each subscale, we performed principal component factor analyses (PFA) with varimax rotation to maximize the loadings and eliminate double loadings. A two-factor solution appeared to be the best fit based on two considerations (10,11). First, the initial design hypothesis for the DSSS was composed of two major subscales: DS and SS. Second, the screen test revealed the possibility of a two or three-factor solution. A loading factor of 0.40 was selected as the item criterion. The internal consistency of the DSSS and its subscales was assessed with Cronbach’s alpha and the item-total correlation. Pearson’s correlations were used to examine the construct validity of the DS and SS against the MADRS and HAMD-S, respectively. The test–retest reliability of the DS, SS and DSSS over 4 weeks was analyzed using the intra-class correlation (ICC) approach. Receiver operating characteristic (ROC) analysis was used to ascertain the cut-off scores for correctly identifying patients in non-full remission (defined as a HAMD score > 7). The areas under the ROC curves (AUCs) were compared by using the Delong Clarke-Pearson method (17). In addition, the sensitivity, specificity, positive predictive value, and negative predictive value of each threshold score were calculated.
Each item of the DSSS deals with only one symptom. Therefore, the number of SS items above “mild” that the subjects were suffering from indicates the number of somatic symptoms of them. To determine whether the DSSS is valuable for evaluating somatic symptoms in patients with depression, the number of somatic symptoms or SS score was compared between in remission and in non-remission patients. Simple linear regression was used to evaluate whether the reported number of somatic symptoms or the SS score could predict the depression severity indicated by the MADRS scores. We analyzed the ROC curve using the MedCalc statistical package (MedCalc Software, Mariakerke, Belgium), and other data were analyzed using the PASW Statistics 20 statistical software (PASW IBM Corp., Armonk, NY, USA).
Ethics statement
The present study protocol was reviewed and approved by the institutional review board of the Korea University Ansan Hospital (IRB No. AS11093). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
Subjects
Two hundred three patients (61 men and 142 women) were included in the analyses. The mean age of the subjects was 45.6 ± 13.1 years. The baseline scores on the MADRS were 14.5 ± 10.4, and those on the HAMD were 13.5 ± 9.5. Ninety-seven (47.8%) showed full remission (HAMD score ≤ 7) at the baseline, and 106 (52.2%) did not. In comparison with patients with non-full remission, patients with full remission had significantly lower scores in the scales of MADRS, HAMD, and DSSS and their subscales at baseline. There were no significant differences in the demographic variables between the groups, with the exception of mean age of subjects (P = 0.02) (Table 1). Among the 203 subjects, 164 (45 men and 119 women, mean age 46.1 ± 12.6 years) completed the required 4-week follow-up visit. The HAMD scores at baseline in 39 subjects (13.9 ± 9.5) who dropped out were not significantly different from those of the 164 follow-up subjects (13.4 ± 9.4).
Table 1. Baseline characteristics of patients.
| Characteristics | Total subjects | Full remission | Non-full remission |
|---|---|---|---|
| Total, No. (%) | 203 (100) | 97 (47.8) | 106 (52.2) |
| Men | 61 (30) | 32 (15.8) | 29 (14.3) |
| Women | 142 (70) | 65 (32.0) | 77 (37.9) |
| Age, mean (SD), yr | 45.6 (13.1) | 47.8 (12.2)* | 43.6 (13.7)* |
| Education level, mean (SD), yr | 10.7 (3.6) | 10.6 (3.4) | 10.8 (3.4) |
| Illness duration, mean (SD), yr | 6.3 (6.0) | 5.7 (5.7) | 6.8 (6.3) |
| MADRS score, mean (SD) | 14.5 (10.4) | 7.1 (5.1) | 21.2 (9.4) |
| HAMD score, mean (SD) | 13.5 (9.5) | 6.3 (3.8) | 20.3 (8.0) |
| DSSS score, mean (SD) | 24.2 (15.7) | 13.5 (10.1) | 34.0 (13.3) |
| DS score | 14.1 (9.4) | 7.6 (5.8) | 20.1 (7.8) |
| SS score | 10.1 (7.1) | 5.9 (5.1) | 13.9 (6.6) |
SD = standard deviation, MADRS = Montgomery-Åsberg Depression Rating Scale, HAMD = Hamilton Depression Rating Scale, DSSS = Depression and Somatic Symptoms Scale, DS = depression subscale, SS = somatic subscale.
*Significant difference between full remission and non-full remission (P < 0.05).
Internal consistency reliability
The Cronbach’s alpha value of the DSSS was 0.95 at baseline. Cronbach’s alpha was 0.91 for the SS and 0.81 for the PS, indicating a high degree of internal consistency. The DS also showed a high degree of internal consistency at 0.93. The correlation coefficients between each item and the total scores of the DS, SS, and PS ranged from 0.48 to 0.80, from 0.54 to 0.76, and from 0.51 to 0.73, respectively. Based on these data, both subscales appear to be internally consistent and have good content validities.
Concurrent validity
In the baseline, the DS scores were correlated with the MADRS scores (r = 0.82, P < 0.001), and the SS scores were correlated with the HAMD-S scores (r = 0.44, P < 0.001). These findings indicate that the DSSS has good concurrent validity.
Temporal stability
Among the 164 follow-up subjects, 147 were long-term patients for at least 3 months, and they were clinically stable patients according to patient self-reports and chart review. The difference in DSSS scores for these 147 subjects between the baseline and the 4-week follow-up was not significant, as indicated by a paired t-test (P = 0.672). The 4-week test-retest stability for the clinically stable 147 patients, as measured by intra-class correlation, was ICC(1,1) = 0.75 (P < 0.001) for the DS, ICC(1,1) = 0.78 (P < 0.001) for the SS, and ICC(1,1) = 0.79 (P < 0.001) for the DSSS.
Factorial validity
Principal component factor analysis with varimax rotation was conducted, and the results are provided in Table 2. The Kaiser-Meyer-Olkin measure of sampling adequacy (0.95) and Bartlett’s test of sphericity (P < 0.001) showed that the data were appropriate for a factor analysis. The two-factor solution accounted for 56.8% of the total variation, with eigenvalues of 11.1 and 1.4, respectively, for the first two factors. Factors 1 and 2 appeared to be the depression factor and the somatic factor, respectively. The DS items had loadings ranging from 0.43 to 0.79, and the SS items had loadings ranging from 0.44 to 0.80 on their corresponding trait factors (Table 2). All items in the SS loaded on Factor 2 with factor loadings > 0.4, although “tightness in the chest,” “dizziness,” and “palpitation” loaded on both factors. All items in the DS loaded on Factor 1 with factor loadings > 0.4.
Table 2. Factor analysis of DSSS using the principal component factoring method with varimax rotation.
| Subscales | Factor 1 | Factor 2 |
|---|---|---|
| Somatic subscale | ||
| 1. Headache* | 0.43 | 0.50 |
| 3. Tightness in the chest | 0.68 | 0.44 |
| 5. Muscle tension | - | 0.74 |
| 7. Back pain* | - | 0.74 |
| 9. Dizziness | 0.52 | 0.46 |
| 11. Chest pain* | 0.46 | 0.49 |
| 13. Neck or shoulder pain* | - | 0.78 |
| 15. Shortness of breath or difficulty breathing | 0.44 | 0.56 |
| 17. Soreness in more than half of the body’s muscles* | - | 0.80 |
| 19. Palpitations or increased heart rate | 0.55 | 0.51 |
| Depression subscale | ||
| 2. Loss of interest in daily or leisure activities | 0.77 | - |
| 4. Insomnia | 0.52 | - |
| 6. Irritable mood | 0.66 | - |
| 8. Unable to feel happy or decreased ability to feel happy | 0.79 | - |
| 10. Depressed mood or tearful | 0.66 | 0.46 |
| 12. Feelings of self-reproach or guilt | 0.74 | - |
| 14. Loss of interest in sex | 0.43 | - |
| 16. Anxious or nervous | 0.73 | 0.40 |
| 18. Unable to concentrate | 0.69 | - |
| 20. Thoughts of death or suicidal ideas | 0.73 | - |
| 21. Fatigue or loss of energy | 0.70 | - |
| 22. Decreased appetite or loss of appetite | 0.69 | - |
DSSS = Depression and Somatic Symptoms Scale.
*Pain subscale.
Cut-off scores for depression (non-full remission)
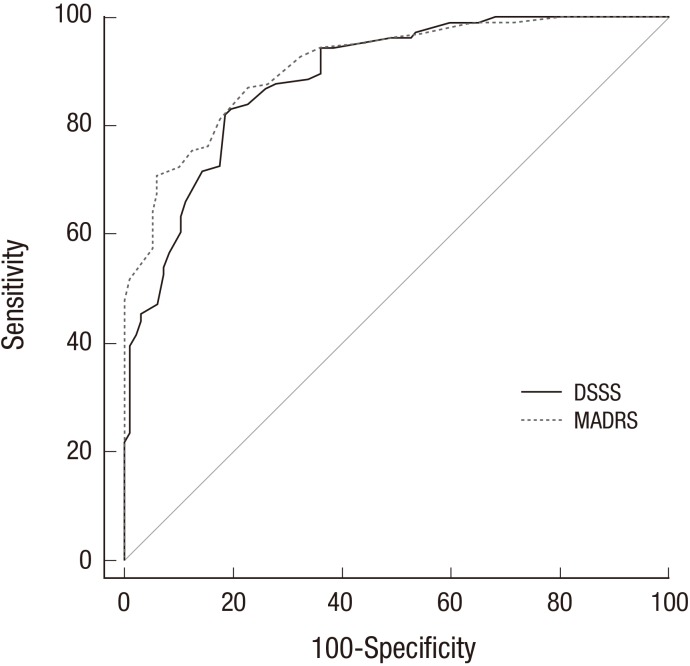
The diagnostic performances of the DSSS and the MADRS are illustrated in Fig. 1. Comparison of the ROC curves demonstrates a statistically nonsignificant diagnostic performance for the DSSS (AUC = 0.887) compared with the MADRS (AUC = 0.911) in the detection of non-full remission. Maximal discrimination between remission and non-full remission was obtained at a cut-off score of 22. This means that a score of 22 or higher is indicative of the presence of non-full remission. When 22 was used as the cutoff value, the sensitivity, the specificity, the PPV, and the negative predictive value (NPV) were 82.1%, 81.4%, 82.9%, and 80.6%, respectively (Table 3).
Fig. 1.
Receiver operating characteristic curve for the DSSS and the MADRS to detect patients with depressed state.
DSSS = Depression and Somatic Symptoms Scale, MADRS = Montgomery-Åsberg Depression Rating Scale.
Table 3. Sensitivity, specificity, PPV, and NPV of the depression and somatic symptoms scale at different cut-off scores for depressed state.
| Score | Sensitivity, % | 95% CI | Specificity, % | 95% CI | PPV, % | NPV, % |
|---|---|---|---|---|---|---|
| > 21 | 83.02 | 74.5–89.6 | 80.41 | 71.1–87.8 | 81.23 | 81.26 |
| > 22 | 82.08 | 73.4–88.8 | 81.44 | 72.3–88.6 | 82.85 | 80.63 |
| > 23 | 81.13 | 72.4–88.1 | 81.44 | 72.3–88.6 | 82.68 | 79.81 |
PPV = positive predictive value, NPV = negative predictive value, CI = confidence interval.
Somatic symptoms evaluated using the DSSS
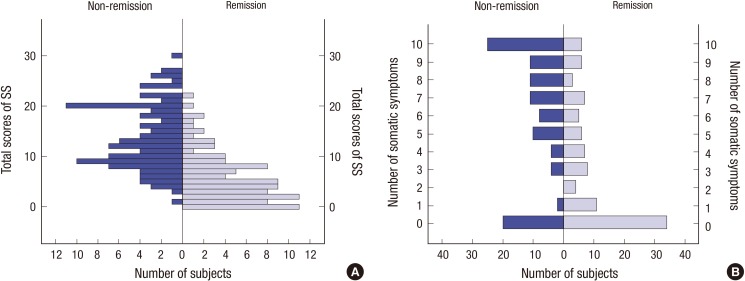
In patients with non-full remission (HAMD score ≤ 7), the mean SS score and mean number of somatic symptoms were 13.9 ± 6.6 and 6.1 ± 3.6, respectively. In patients with full remission, the mean SS score and mean number of somatic symptoms were 5.9 ± 5.1 and 3.3 ± 3.4, respectively. The SS scores and the number of somatic symptoms in non-remission patients were greater than those in remission patients (both P < 0.001) (Fig. 2).
Fig. 2.
The SS scores and number of somatic symptoms evaluated using the DSSS in subjects with non-remission (n = 106) and in subjects with full remission (n = 97). (A) Frequencies of number of subjects depending on SS score in non-remission and in remission. (B) Frequencies of number of subjects depending on number of somatic symptoms in non-remission and in remission.
DSSS = Depression and Somatic Symptoms Scale, SS = somatic subscale.
Ability of somatic symptoms to predict depression severity
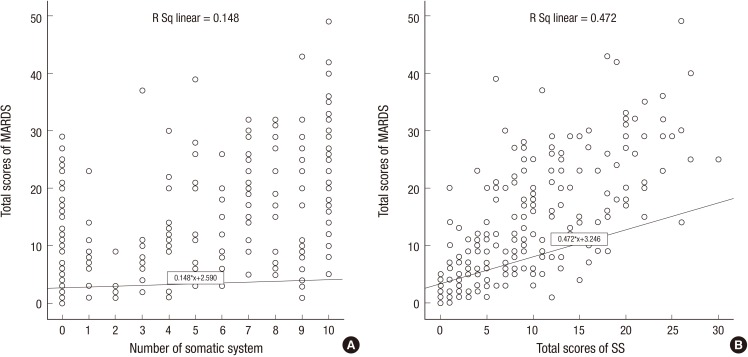
For all the patients, we used simple linear regression to assess whether the reported number of somatic symptoms or the SS scores could predict the depression severity as indicated by the MADRS scores. The slope of the regression line for the number of somatic symptoms was greater than zero, indicating that the MADRS scores tended to increase as the number of somatic symptom increased (slope: 0.148, t201: 6.32, P < 0.001, y: 2.590 + 0.148x, R[2] = 0.16). The slope of the regression line for the SS scores was greater than zero, indicating that the MADRS scores tended to increase as the SS score increased (slope: 0.472, t201: 13.6, P < 0.001, y: 3.246 + 0.472x, R[2] = 0.479) (Fig. 3).
Fig. 3.
Simple linear regression (n = 203) for evaluating whether the number of somatic symptoms or the SS scores predict the MADRS scores. These findings reach statistical significance in linear regression analysis (both P < 0.001). (A) Linear regression of the number of somatic symptoms on the MADRS scores. (B) Linear regression of the SS scores on the MADRS scores.
MADRS = Montgomery-Åsberg depression rating scale, SS = somatic subscale.
DISCUSSION
Overall, the Korean version of the DSSS retains the psychometrically sound characteristics of the original scale. The DSSS and each subscale (DS, SS, and PS) were internally consistent and had good content validities. The results of the factor analysis corresponded with those of a previous study (11), confirming that the DSSS has a two-factor structure (depression and somatic factors). In this study, all vegetative symptoms belonged to the depression factor. Vegetative symptoms are considered closer to the depression dimension than the somatic dimension. As a result, vegetative symptoms are included in the DSM-IV criteria for MDD and depression measurements such as the HAMD and MADRS (12,13,18). In this context, the original version of the DSSS also included vegetative symptoms in the DS (11).
To investigate its concurrent validity, the DS was compared with the MADRS, and the SS with the HAMD-S, because the MADRS and the HAMD differ in their levels of measuring the somatic dimension (19). In the MADRS, cognitive symptoms of depression are more emphasized than somatic symptoms in a simple factor structure (20). Other than the two vegetative symptom items, no somatic symptoms are included in the MADRS. Accordingly, the DS was compared with the MADRS, which has a minimized somatic dimension. In contrast, in the HAMD, somatic symptoms and vegetative symptoms are more emphasized than in the MADRS, in a multi-faceted factor structure (21). The 5 items in the HAMD, excluding the 3 vegetative symptoms, are considered as a somatic dimension. In this study, the HAMD-S, composed of these 5 items, was compared with the SS. Both the DS and the SS showed satisfactory concurrent validity.
On the follow-up visit, 147 patients who had received treatment for at least 3 months and were confirmed to have been clinically stable were analyzed to investigate the test-retest reliability. These patients in long-term ongoing treatment might be less symptomatic and less sensitive to symptom change during the test-retest period. It is most desirable to conduct a retest within one week of the initial test. However, it was conducted 4 weeks later in this study to match the typical return of clinically stable patients in the clinical setting. Even with this 4-week limitation, the test–retest stability was relatively high. The difference in the DSSS score between at baseline (24.5 ± 15.9) and at the second visit (22.9 ± 16.0) was not statistically significant (P = 0.674). These values indicate that the symptoms of the 146 patients had somewhat improved. Accordingly, the ICC (DSSS: 0.79, DS: 0.75, and SS: 0.78) of this study was slightly lower that in the original-version ICC (DSSS: 0.92, DS: 0.88, and SS: 0.90) (11).
The AUC for identifying patients in non-full remission showed a high diagnostic accuracy in both the MADRS (0.911) and the DSSS (0.887). The scales are known to show slightly differential patterns of correlations, depending on the method of collecting the data (self-reported vs. clinician rated) (22). The AUC of the MADRS, which is a clinician-administered instrument, was slightly greater than that of the self-reported DSSS, although the difference was statistically insignificant. This may be because discrimination of remission was defined by the clinician-administered HAMD.
At a cut-off score of 22, the sensitivity and specificity levels were 82.1% and 81.4%, respectively, where the sum of sensitivity and specificity becomes the maximum. At this score or higher, the sensitivity level is higher than the specificity level. Both sensitivity and specificity must be considered because non-full remission is confirmed during the depression treatment process (23). The purpose for developing the DSSS is to assess the effects of somatic symptoms on the course of depression, rather than to screen for depression (10).
The number of somatic symptoms represents the range of somatic symptoms, and the SS score represents the severity of somatic symptoms. The number of somatic symptoms and the SS score significantly differed depending on the presence of depression remission. The number of somatic symptoms in non-remission patients might be greater than in remission patients because somatic symptoms still remain as residual symptoms even after the depression has been treated (5). Alternately, a large number of somatic symptoms may have inhibited the remission of depression (24). The depression severity indicated by the MADRS score could be significantly predicted by the number of somatic symptoms or the SS score. In other words, both the range and severity of somatic symptoms have a great ability to predict the severity of depression. This result supports that the SS items were properly developed and selected as items reflecting the severity of depression.
The DSSS is expected to be very useful in the consulting field with medical/surgical departments, as well as in psychiatric clinics. Consulting patients may frequently have somatic symptoms accompanying depression. Patients with medical/surgical problems have various physical limitations, and clinicians have time restrictions. Accordingly, it is difficult to conduct the first psychiatric examination in detail, as well as apply multiple scales. The DSSS may be the best choice for these situations. Moreover, non-psychiatrists can use the DSSS with their patients because it does not require specific training.
The followings are limitations or topics to be carefully interpreted in this study. 1) This study was carried out in a single-territory hospital. Only the outpatients of ages from 18 to 65 years old were targeted. The majority of the patients were women and in their 40s and 50s. Further studies validating different sociodemographics (e.g., elderly patients) or different clinical characteristics (e.g., other medical/surgical patients) are recommended in the future. 2) Patients were diagnosed on the basis of an unstructured clinical interview. However, this is a common feature of studies conducted within a usual clinical practice. 3) In the construct validity and AUC analyses, the self-rated DSSS was compared with clinician-rated scales such as the HAMD and MADRS. 4) Regarding concurrent validity, the SS could not be compared with the other somatic scales, such as the PHQ-15 and SCL-90. 5) A HAMD score of 7 or less was defined as remission. Although this score of 7 is widely adopted as standard, some researchers suggested that it is too high (25,26). 6) There was no control group because of observational research design.
The Korean version of the DSSS, which was confirmed to have a two-factor structure, showed satisfactory evidence for both good reliability and good validity. The DSSS is an excellent screening instrument of symptomatic remission, and a score of 22 was suggested as a cut-off score identifying non-full remission. It was confirmed that both the number and severity of somatic symptoms have a significant effect on depression, especially predicting severity of depression and discriminating between remission and non-remission patients with depression. The DSSS is composed of somatic items that have significant impacts on depression, and it may overcome the deficiency of other depression scales that include few somatic symptoms. In summary, this study confirmed the DSSS as a useful instrument that can measure both depression and somatic symptoms simultaneously, rapidly, and accurately. These features of the DSSS would be useful in clinical practice settings and in consultation fields.
Footnotes
Funding: This research was supported by a grant of the Korea Mental Health Technology R & D Project, Ministry of Health and Welfare, Republic of Korea (grant number: HM15C1223), and was partially supported by Hanlim Pharmaceuticals, Seoul, Republic of Korea.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception and design: Jeon SW, Ko YH. Acquisition, analysis, or interpretation of data: Jeon SW, Yoon SY, Ko YH, Joe SH, Kim YK, Han C, Yoon HK. Drafting of the manuscript: Jeon SW, Ko YH. Critical revision of the manuscript: Liu C. Receiving grant: Ko YH. Approval of final manuscript: all authors.
References
- 1.Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341:1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- 2.Park SC, Sakong J, Koo BH, Kim JM, Jun TY, Lee MS, Kim JB, Yim HW, Park YC. Clinical significance of the number of depressive symptoms in major depressive disorder: results from the CRESCEND study. J Korean Med Sci. 2016;31:617–622. doi: 10.3346/jkms.2016.31.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greden JF. Physical symptoms of depression: unmet needs. J Clin Psychiatry. 2003;64(Suppl 7):5–11. [PubMed] [Google Scholar]
- 4.Leuchter AF, Husain MM, Cook IA, Trivedi MH, Wisniewski SR, Gilmer WS, Luther JF, Fava M, Rush AJ. Painful physical symptoms and treatment outcome in major depressive disorder: a STAR*D (sequenced treatment alternatives to relieve depression) report. Psychol Med. 2010;40:239–251. doi: 10.1017/S0033291709006035. [DOI] [PubMed] [Google Scholar]
- 5.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 6.Tylee A, Gandhi P. The importance of somatic symptoms in depression in primary care. Prim Care Companion J Clin Psychiatry. 2005;7:167–176. doi: 10.4088/pcc.v07n0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fava M. Depression with physical symptoms: treating to remission. J Clin Psychiatry. 2003;64(Suppl 7):24–28. [PubMed] [Google Scholar]
- 8.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins symptom checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Hung CI, Weng LJ, Su YJ, Liu CY. Preliminary study of a scale measuring depression and somatic symptoms. Psychol Rep. 2006;99:379–389. doi: 10.2466/pr0.99.2.379-389. [DOI] [PubMed] [Google Scholar]
- 11.Hung CI, Weng LJ, Su YJ, Liu CY. Depression and somatic symptoms scale: a new scale with both depression and somatic symptoms emphasized. Psychiatry Clin Neurosci. 2006;60:700–708. doi: 10.1111/j.1440-1819.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahn YM, Lee KY, Yi JS, Kang MH, Kim DH, Kim JL, Shin J, Shin HK, Yeon BK, Lee JH, et al. A validation study of the Korean-version of the Montgomery-Asberg depression rating scale. J Korean Neuropsychiatr Assoc. 2005;44:466–476. [Google Scholar]
- 15.Yi JS, Bae SO, Ahn YM, Park DB, Noh KS, Shin HK, Woo HW, Lee HS, Han SI, Kim YS. Validity and reliability of the Korean version of the Hamilton depression rating scale (K-HDRS) J Korean Neuropsychiatr Assoc. 2005;44:456–465. [Google Scholar]
- 16.Zimmerman M, Chelminski I, Posternak M. A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis. 2004;192:595–601. doi: 10.1097/01.nmd.0000138226.22761.39. [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, D.C.: American Psychiatric Association; 1995. [Google Scholar]
- 19.Khan A, Brodhead AE, Kolts RL. Relative sensitivity of the Montgomery-Asberg depression rating scale, the Hamilton depression rating scale and the clinical global impressions rating scale in antidepressant clinical trials: a replication analysis. Int Clin Psychopharmacol. 2004;19:157–160. doi: 10.1097/00004850-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D, Woo A, Trivedi MH. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol. 2006;16:601–611. doi: 10.1016/j.euroneuro.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pancheri P, Picardi A, Pasquini M, Gaetano P, Biondi M. Psychopathological dimensions of depression: a factor study of the 17-item Hamilton depression rating scale in unipolar depressed outpatients. J Affect Disord. 2002;68:41–47. doi: 10.1016/s0165-0327(00)00328-1. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman M, McGlinchey JB. Depressed patients’ acceptability of the use of self-administered scales to measure outcome in clinical practice. Ann Clin Psychiatry. 2008;20:125–129. doi: 10.1080/10401230802177680. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman M, McGlinchey JB, Posternak MA, Friedman M, Attiullah N, Boerescu D. How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry. 2006;163:148–150. doi: 10.1176/appi.ajp.163.1.148. [DOI] [PubMed] [Google Scholar]
- 24.Karp JF, Scott J, Houck P, Reynolds CF, 3rd, Kupfer DJ, Frank E. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66:591–597. doi: 10.4088/jcp.v66n0508. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman M, Posternak MA, Chelminski I. Is the cutoff to define remission on the Hamilton rating scale for depression too high? J Nerv Ment Dis. 2005;193:170–175. doi: 10.1097/01.nmd.0000154840.63529.5d. [DOI] [PubMed] [Google Scholar]
- 26.Romera I, Pérez V, Menchón JM, Polavieja P, Gilaberte I. Optimal cutoff point of the Hamilton rating scale for depression according to normal levels of social and occupational functioning. Psychiatry Res. 2011;186:133–137. doi: 10.1016/j.psychres.2010.06.023. [DOI] [PubMed] [Google Scholar]