FIGURE 6.
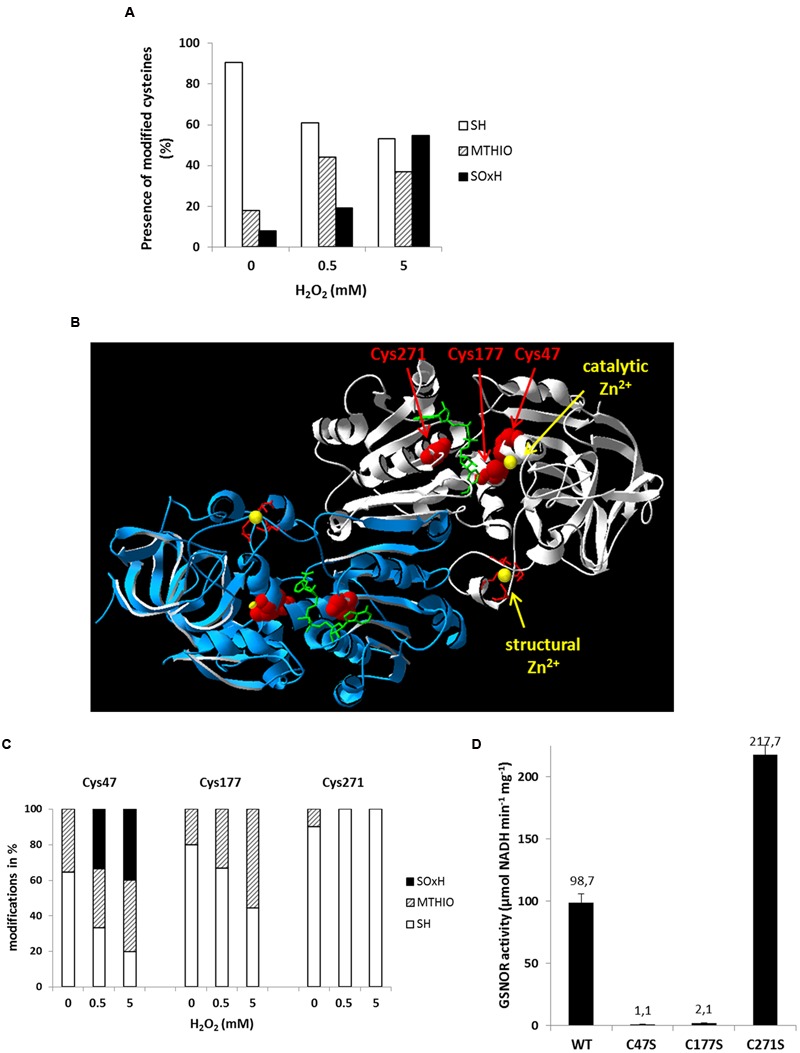
Oxidative modifications of multiple cysteine residues of GSNOR correlate to reduced activity. (A) nano LC-MS/MS analysis of cysteine residues of recombinant GSNOR oxidized with 0.5 and 5 mM H2O2. The portion of different modification is represented as the mean percentage of all detected peptides (modifications of individual cysteines are shown in Supplementary Figure S1). SH represents free cysteines, MTHIO-labeling shows reversibly modifications, and SOxH represents irreversibly oxidative modifications. (B) Percentage distribution of different modifications (SH, MTHIO, and SOxH) of Cys47, Cys177, and Cys271 residues of oxidized GSNOR by nano LC-MS/MS. (C) 3D structure of Arabidopsis GSNOR (PDB code: 3UKO) as a homodimer (two subunit is labeled by white and blue). Cysteine residues in the substrate-binding site are highlighted in red. The bound NAD+ cofactor is shown by green sticks. The catalytic and structural Zn2+ is labeled by yellow. (D) Specific enzyme activity was determined of WT GSNOR and cysteine mutants GSNORC47S, GSNORC177S, and GSNORC271S. The mean values with SD of three determinations are presented in the graph.
