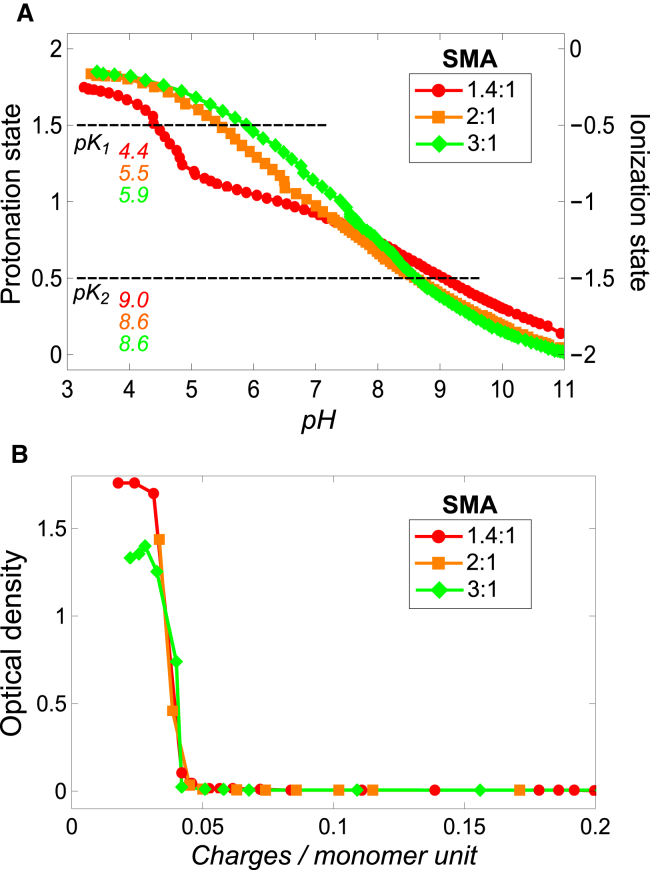
Figure 3.
Influence of ionization state on aqueous solubility of SMA. (A) Protonation state (left axis) and corresponding ionization state (right axis) of the monomol of three SMA variants. The monomol is defined as the smallest unit of the polymer that represents its overall monomer composition. Titrations were performed in triplicate and gave very similar results. From the three repeated experiments the maximum error in pKa values is estimated to be ±0.2. For clarity, only a single representative ionization curve is shown for each SMA variant. (B) Aqueous solubility of the SMA variants as function of the linear charge density, which is given as the number of charges per monomer unit where a monomer unit represents either maleic acid or styrene. This graph was prepared by combining the results that are shown in Figs. 2A and 3A. Solid lines were added to guide the eye. To see this figure in color, go online.