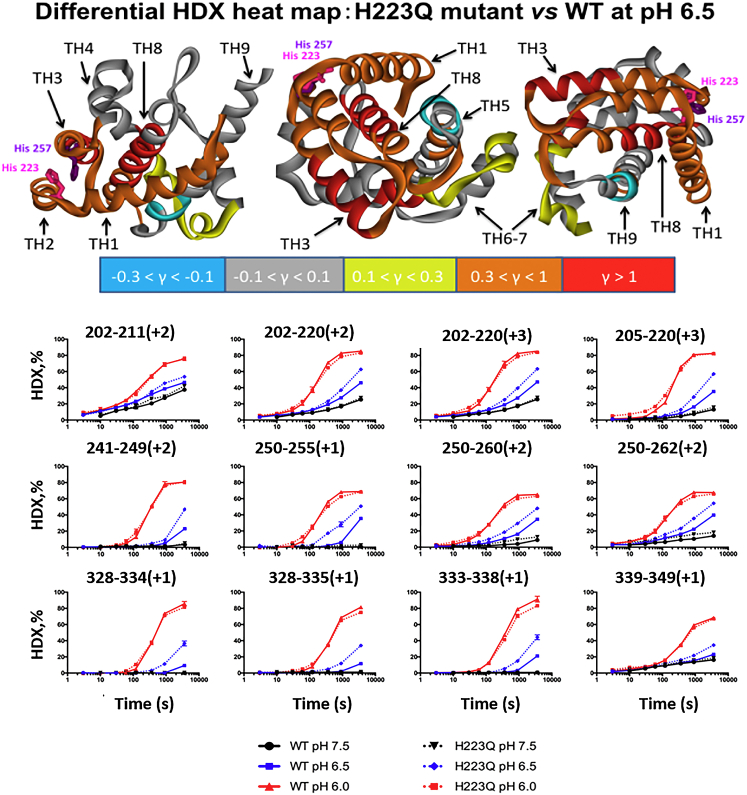
Figure 3.
HDX measurements of the conformational flexibility of the T domain WT and H223Q mutant. (Bottom) Kinetic HDX curves of proteolytic peptides measured at pH 7.5, 6.5, and 6.0. All time points were corrected to standard condition at pH 7.5 and 4°C, and measurements were conducted as described in (46). The title of each data subplot refers to the amino acid residue number and charge state of the peptide in the mass spectrometer. Both proteins have equally low exchange at pH 7.5 and equally high exchange at pH 6.0. At the intermediate pH value of 6.5, the exchange in H223Q is noticeably greater than that in the WT. (Top) Differential heat maps of the HDX of H223Q versus WT at pH 6.5. The ribbon diagrams are colored according to the relative differential uptake factor, γ = (HDXH223Q − HDXWT)/HDXWT, measured for the last kinetic point of 3000 s (see key below heat maps). The three versions of the map represent three different orientations of the structure. The highest differential HDX is observed for amphipathic helixes TH1–3 and for the central hydrophobic helix, TH8. To see this figure in color, go online.