Abstract
Objective
Ultrafast and whole-body cooling can be induced by total liquid ventilation (TLV) with temperature-controlled perfluorocarbons. Our goal was to determine whether this can afford maximal cardio- and neuroprotections through cooling rapidity when coronary occlusion is complicated by cardiac arrest.
Design
Prospective, randomized animal study.
Setting
Academic research laboratory.
Subjects
Male New-Zealand rabbits.
Interventions
Chronically instrumented rabbits were submitted to coronary artery occlusion and ventricular fibrillation. After 8-min of cardiac arrest, animals were resuscitated and submitted to a normothermic follow-up (Control group) or to 3-h of mild hypothermia induced by TLV (TLV group) or by combination of cold saline infusion and cold blankets application (Saline group). Coronary reperfusion was permitted 40-min after the onset of occlusion. After awakening, rabbits were followed during 7 days.
Measurements and main results
Ten animals were resuscitated in each group. In the Control group, all animals secondarily died from cardiac/respiratory failure (8/10) or neurological dysfunction (2/10). In the Saline group, the target temperature of 32°C was achieved within 30–45 min after cooling initiation. This slightly reduced infarct size vs Control (41±16% vs 54±8% of risk zone, respectively; p<0.05) but failed to significantly improve cardiac output, neurological recovery and survival rate (3 survivors, 6 death from cardiac/respiratory failure and 1 from neurological dysfunction). Conversely, the 32°C temperature was achieved within 5–10 min in the TLV group. This led to a dramatic reduction in infarct size (13±4%; p<0.05 vs other groups) and improvements in cardiac output, neurological recovery and survival (8 survivors, 2 deaths from cardiac/respiratory failure).
Conclusions
Achieving hypothermia rapidly is critical to improve the cardiovascular outcome after cardiac arrest with underlying myocardial infarction.
Keywords: Animals; Cardiac Output; Nervous System Diseases; Prospective Studies; Rabbits; Random Allocation; Survival Rate; Time Factors; Cardiopulmonary Resuscitation; Coronary Occlusion; Heart Arrest; Heart Rate; Hypothermia, Induced; Liquid Ventilation; Male; Myocardial Infarction
Introduction
Out-of-hospital cardiac arrest is a leading cause of death in the world. After cardiopulmonary resuscitation, comatose survivors require integrated post-resuscitation care (1). Among the recommended treatments, hypothermia provides neuroprotection (2) while percutaneous coronary interventions is intended for revascularization during coronary artery occlusion (CAO) (3). Since hypothermia also decreases myocardial susceptibility to ischemia (4–6), it could potentiate the cardioprotective effect of reperfusion therapies and further improve hemodynamics when cardiac arrest is associated with coronary obstruction. This cardiac benefit could however be compromised if hypothermia is only achieved into the coronary reperfusion phase (4–6), supporting the importance of cooling the heart quickly. This importance has also been evidenced for the neurological effect of hypothermia in animal models of global (7–10) and brain (11) ischemia. Here, we hypothesize that rapidity of cooling is also critical to achieve maximal protection and to prevent cardiovascular mortality when cardiac arrest is combined with CAO.
In order to investigate the importance of the cooling rate, we previously studied ultra-fast cooling through total liquid ventilation (TLV). It consists in the tidal ventilation of lungs with temperature-controlled perfluorocarbons that can cool the body while maintaining gas exchanges (12). After global ischemia, TLV provided a greater neurological benefit and survival improvement than a conventional cooling in a rabbit model (12). The superiority of TLV was also shown regarding infarct size reduction in rabbits submitted to CAO (5, 6, 13). Importantly, these previous studies were conducted in pure models of cardiac arrest or CAO but they did not reproduce the complexe interactions occuring in vivo during their combination, as often observed in patients (1, 3).
In the present study, we aimed to determine whether cooling rapidity, assessed by comparison of TLV with a conventional “load-dependent” hypothermia (cold saline infusion plus external cooling), could be actually critical to achieve maximal cardiac, neurological and survival benefits after combined cardiac arrest and CAO in chronically instrumented rabbits.
Materials and Methods
The experiments were conducted in accordance with French official regulations, after approval by the local ethical review board. They conformed to the “Position of the American Heart Association on research animal use”.
Animal preparation
Male New-Zealand rabbits were anesthetized using zolazepam, tiletamine and pentobarbital (all 20–30 mg/kg i.v.). They were chronically instrumented with a peri-coronary pneumatic occluder, an aortic cardiac output probe (PS-Series Probes, Transonic, NY, USA) and an electrode upon the chest.
Coronary occlusion, cardiac arrest and cardiopulmonary resuscitation
Two weeks after surgery, animals were reanesthetized, intubated and mechanically ventilated (Fi02=100%; Vt=10 ml/kg; 26 breaths/min). The peri-coronary occluder was inflated to induce CAO. Two minutes later, ventricular fibrillation was induced by passing an alternating current (10 V, 4 mA) between the chest electrode and another electrode within the esophagus. After 8 min of untreated fibrillation, cardiopulmonary resuscitation was started using manual lateral chest compressions (~200 compressions/min), electric attempts of defibrillation (5–10 J/kg) and intravenous administration of epinephrine (15 μg/kg i.v.). Mechanical ventilation was stopped throughout the cardiac arrest period and restarted at the onset of cardiopulmonary resuscitation. Resumption of spontaneous circulation (ROSC) was considered as an organized cardiac rhythm associated with a mean arterial pressure above 40 mmHg during at least 1 min. Coronary reperfusion was permitted through occluder deflation after 40 min of CAO. After ROSC, administration of epinephrine was permitted during 7 h to maintain mean arterial pressure at ~80 mmHg. Mechanical ventilation was continued until awakening of the animals.
Experimental protocol
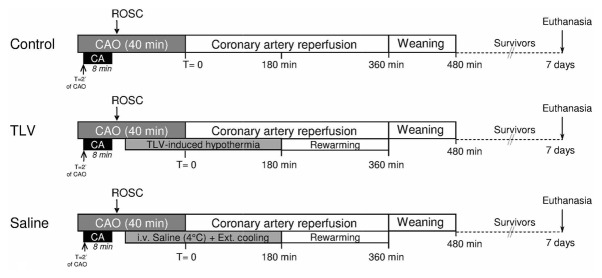
After ROSC, rabbits randomly underwent life support under normothermic conditions (Control group) or with therapeutic hypothermia (TLV and Saline groups)(Figure 1). In the TLV group, hypothermia was induced by 20 min of liquid ventilation after filling the lungs with 10 ml/kg of perfluorodecalin, as previously described (5, 6, 12, 13). The liquid ventilator was set initially to a respiratory rate of 6 breaths/min and a tidal volume of ~7–10 ml/kg. If necessary, ventilatory parameters were adjusted to maintain saturation of peripheral oxygen (SpO2) over 90%. The temperature of perfluorocarbon was initially set at 20°C and progressively increased to 32°C to maintain oesophageal temperatures at ~32°C. After 20 min of TLV, animals resumed to conventional gas ventilation. In the Saline group, hypothermia was induced by cold blankets over the skin and infusion of cold (4°C) saline (NaCl 0.9%, 30 ml/kg i.v. over 30 min). In both Saline and TLV groups, hypothermia was maintained externally at 32°C during 3 h after the onset of the cooling protocol. Subsequently, animals were actively rewarmed using thermal pads and infrared lights until weaning from conventional ventilation and awakening.
Figure 1.
Schematic representation of the experimental protocol.
CAO, coronary artery occlusion; CA, cardiac arrest; ROSC, Resumption of spontaneous circulation; TLV, Total Liquid Ventilation; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling
Investigated parameters
Hemodynamic parameters were continuously recorded using external electrocardiogram, blood pressure in the ear artery and cardiac output flowmeter (Flowmeter-T206, Transonic, NY, USA).
Throughout the 7 days following cardiac arrest, the animals underwent a regular survival and clinical follow-up. Neurological dysfunction was assessed using a previously described score system (0–10% = normal; 100% = brain death) (12, 14). In accordance with the ethical committee, the animals showing a neurological dysfunction score above 60% during 48 hours were euthanized and considered as dead from neurological dysfunction. At the end of the 7 days of follow-up, surviving rabbits were reanesthetized and a pressure catheter (SciSense, London, Ontario, Canada) was introduced into the left ventricle for measurement of end-diastolic pressure and left ventricular rate of pressure development. These parameters were also measured in a group of Sham rabbits neither submitted to cardiac arrest nor hypothermia.
Post-mortem analysis
Seven days after cardiac arrest, surviving animals were euthanized for organ sampling. Pathological analyses were also conducted in the animals that died prematurily. In the heart, risk zone and infarct sizes were determined using triphenyltetrazolium chloride staining (5, 13). In all organs, the lesion severity was assessed by histology and quantified using a 0–3 score system (12). For the brain, a neuropathologist semi-quantitatively and blindly assessed the amount of ischemic neurons in the cortex, hippocampus and cerebellum, as previously described (12). The brain score was the mean value obtained for these three areas. For lungs, we assessed two separate scores for cardiogenic lesions (serous edema and/or congestion) and infectious complication of bronchopneumonia, respectively (12). In the myocardium, these analyses were performed in a remote area, out of the risk zone.
Statistical analyses
Data were expressed as mean±SD. The normality of data distribution was tested using the Kolmogorov-Smirnov analysis. For normally distributed parameters, comparisons were made between groups using a one-way ANOVA at selected time-points as the number of animals varied among time. Post-hoc analyses were performed using the Fisher Least Significant Difference Method. For non-normally distributed parameters, neurological dysfunction and histological scores, comparisons were made using a non-parametric Kruskall-Wallis test followed by a Mann-Whitney analysis. Survival curves were obtained using a Kaplan-Meier analysis and compared between group using a log-rank test. Significant differences were determined at P≤0.05.
Results
Out of the 40 rabbits submitted to cardiac arrest, 30 animals were successfully resuscitated and included in the final experimental groups (n=10/group). The time to ROSC was 2.9±0.8, 2.8±1.4 and 3.1±1.4 min in Control, TLV and Saline groups, respectively. Temperatures decreased very rapidly after the onset of cooling in TLV as compared to Saline group (Figure 2).
Figure 2.
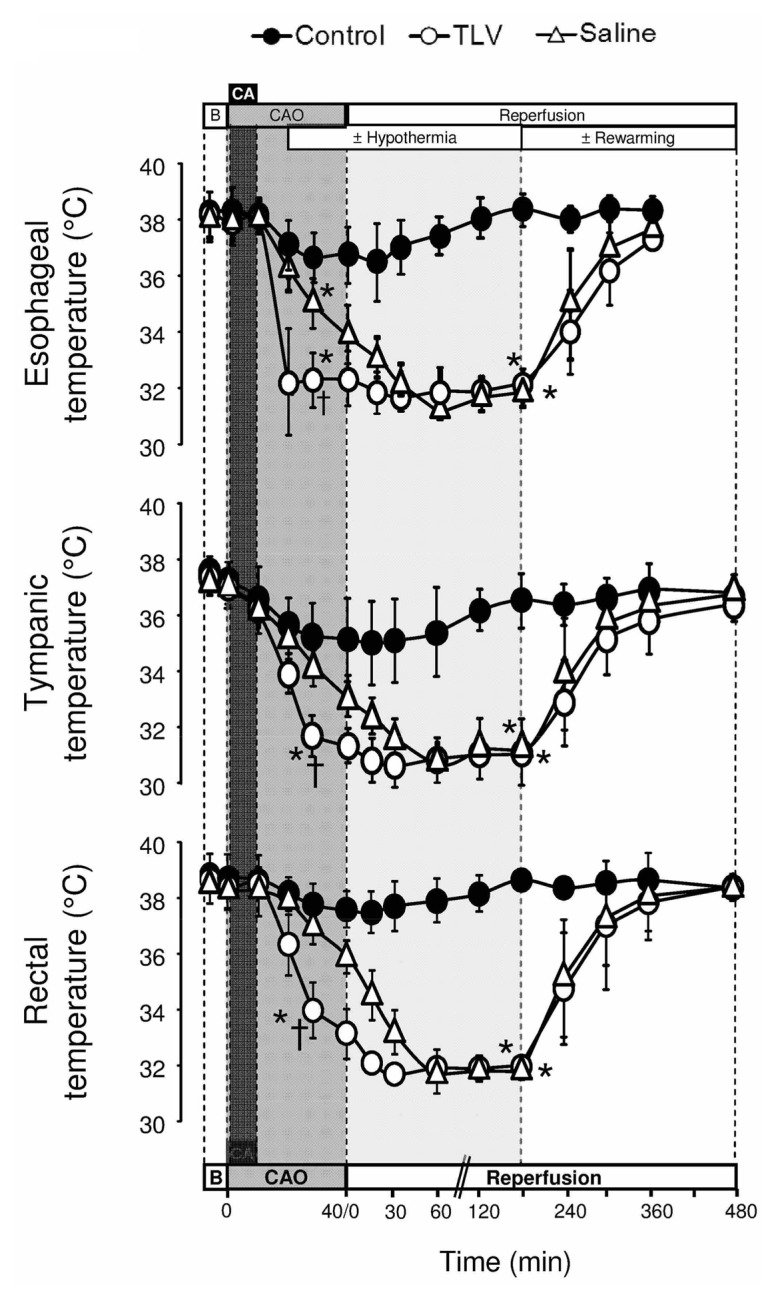
Esophageal, tympanic and rectal temperatures.
Statistical analyses was performed at baseline (B; n=10 in each group), at t=30 min of CAO (n=10 in each group), at t=180 min of reperfusion (n=9, 10 and 9 in Control, TLV and Saline, respectively) and t=480 min of reperfusion (n=7, 9 and 8 in Control, TLV and Saline, respectively). See legend of Figure 1; *, p<0.05 vs corresponding Control; †, p<0.05 vs corresponding Saline.
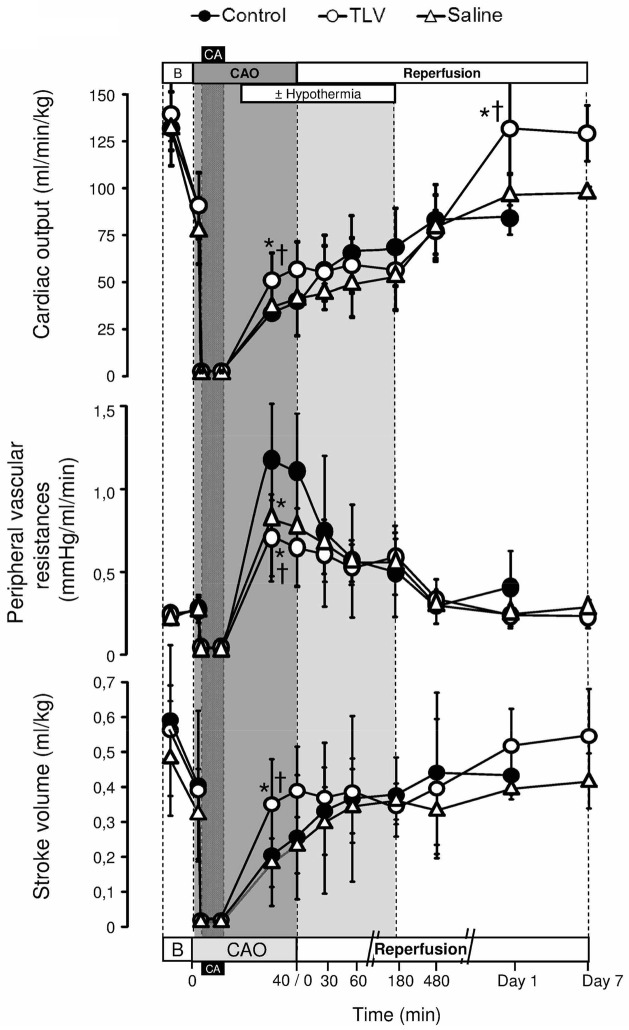
TLV prevents early hemodynamic dysfunction
As illustrated in Figure 3, TLV reduced left ventricular dysfunction after resuscitation. After 30 min of CAO, cardiac output and stroke volumes were indeed increased in TLV vs Control and Saline groups. Heart rate was conversely decreased throughout hypothermia in both TLV and Saline groups (Table I). One day after cardiac arrest, cardiac output was again increased in survivors of the TLV group as compared to Control and Saline groups. Hypothermia also decreased the amount of epinephrine required to maintain mean arterial pressure at ~80 mmHg after cardiac arrest (336±209, 305±153 vs 751±488 μg/kg in TLV and Saline vs Control groups, respectively; p<0.05). Troponin I blood release was also dramatically attenuated in TLV vs Control and Saline groups (Table II).
Figure 3.
Cardiac output, peripheral vascular resistances and left ventricular stroke volume.
See legend of Figure 2.
Table I.
Heart rate and mean arterial pressure
| Baseline | CAO | Coronary reperfusion | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 min | 30 min | 180 min | 480 min | Day 1 | Day 7 | ||
| Number of animals (n) | |||||||
| Control | 10 | 10 | 10 | 9 | 8 | 4 | 0 |
| TLV | 10 | 10 | 10 | 10 | 9 | 9 | 8 |
| Saline | 10 | 10 | 10 | 9 | 9 | 6 | 3 |
| Heart rate (beats/min) | |||||||
| Control | 253±25 | 241±24 | 205±21 | 224±21 | 256±25 | 263±67 | - |
| TLV | 257±25 | 234±30 | 145±19*† | 161±21* | 223±36 | 264±45 | 242±34 |
| Saline | 255±34 | 231±30 | 197±34 | 148±14* | 242±30 | 255±50 | 241±47 |
| Mean arterial pressure (mmHg) | |||||||
| Control | 78±11 | 60±16 | 89±12 | 79±9 | 78±8 | 70±15 | - |
| TLV | 80±13 | 60±12 | 82±10 | 83±4 | 74±8 | 72±6 | 68±14 |
| Saline | 77±9 | 55±11 | 81±5 | 79±5 | 77±9 | 77±12 | 74±14 |
TLV, total liquid ventilation; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling;
, p<0.05 vs corresponding Control;
, p<0.05 vs corresponding Saline.
Table II.
Troponin I, creatinine and alanine-aminotransferase (ALAT) blood levels
| Baseline | Coronary reperfusion
|
|||
|---|---|---|---|---|
| 60 min | 360 min | Day 1 | ||
| Number of animals (n) | ||||
| Control | 9 | 9 | 8 | 4 |
| TLV | 10 | 10 | 9 | 9 |
| Saline | 10 | 9 | 8 | 7 |
| Troponine I blood levels (ng/ml) | ||||
| Control | < 0.2 | 151±62 | 147±58 | 111±50 |
| TLV | < 0.2 | 8±11 *† | 75±83 | 28±40 *† |
| Saline | < 0.2 | 61±27 * | 152±62 | 91±46 |
| Creatinine plasma levels (mg/l) | ||||
| Control | 5.9±1.3 | 9.3±2.2 | 8.5±2.9 | 9.4±5.2 |
| TLV | 6.8±1.6 | 7.8±2.8 | 7.3±1.7 | 8.5±2.8 |
| Saline | 7.4±2.7 | 6.4±3.5 | 5.2±2.9 | 6.1±3.2 |
| Alanine-aminotransferase plasma levels (UI/l) | ||||
| Control | 26±13 | 74±36 | 79±48 | 79±28 |
| TLV | 33±20 | 59±39 | 87±64 | 83±78 |
| Saline | 28±9 | 61±33 | 83±58 | 61±29 |
TLV, total liquid ventilation; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling;
, p<0.05 vs corresponding Control;
, p<0.05 vs corresponding Saline.
TLV improves cardiovascular outcome and provides cardioprotection
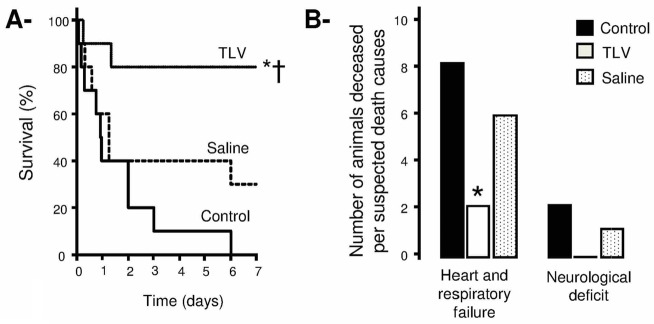
As shown in Figure 4 (Panels A–B), the hemodynamic improvements observed in the TLV group was associated with a reduced mortality, in particular from cardiovascular and/or respiratory failures. Seven days after cardiac arrest, the survival rate achieved 80% in TLV group vs 0% and 30% in Control and Saline groups, respectively. As shown in Table III, left ventricular performance was not altered in survivors of the TLV group as compared to Sham animals, suggesting a complete recovery. Conversely, a pattern of heart failure was observed in the 2 surviving rabbits of the Saline group.
Figure 4.
Panel A: Kaplan-Meyer survival curves throughout the 7 days following coronary artery occlusion and cardiac arrest in the different experimental groups.
Panel B: Number of animals deceased before 7 days of survival follow-up in each group, according the suspected cause of death (cardiogenic vs neurological death).
See legend of Figure 2.
Table III.
Hemodynamic parameters one week after cardiac arrest in surviving animals. In order to determine “usual” values, parameters were also assessed in Sham rabbits not submitted to cardiac arrest.
| Control | TLV | Saline | Sham | |
|---|---|---|---|---|
| Number of animals (n) | 0 | 8 | 2 | 8 |
| Left ventricular maximal rate of pressure development ( dP/dt max) | - | 4908±1130 | 1848±815 | 5535±2318 |
| Left ventricular minimal rate of pressure development ( dP/dt min) | - | 4270±1166 | −1317±499 | 5339±1376 |
| Left ventricular maximal systolic pressure (mmHg) | - | 102±20 | 60±4 | 97±17 |
| End-diastolic left ventricular pressure (mmHg) | - | 6±2 | 14±6 | 4±1 |
TLV, total liquid ventilation; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling;
, p<0.05 vs corresponding Control;
, p<0.05 vs corresponding Saline.
Statistical comparisons were only performed between TLV and Sham groups (no difference); One rabbit died in the Saline group during anaesthesia before catheterization.
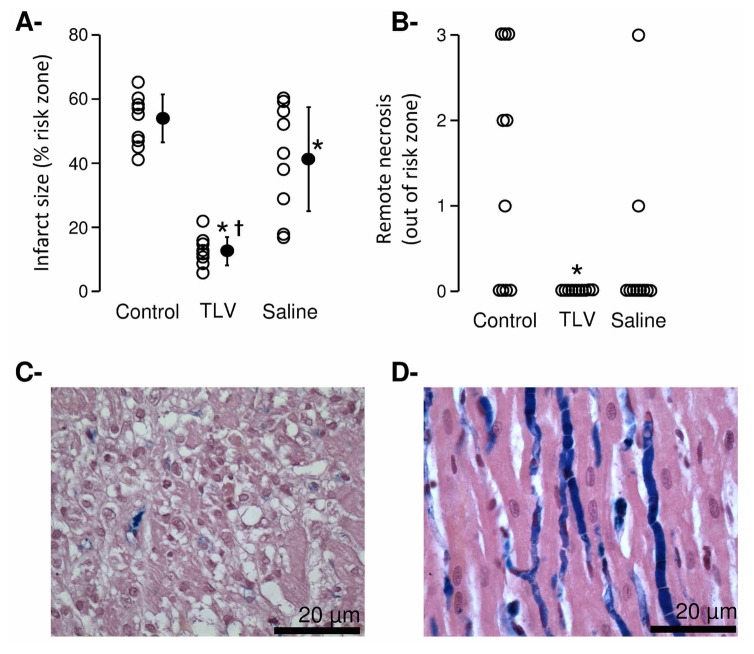
Despite similar risk zone sizes in all groups (35±7, 34±6 and 40±6 % of the left ventricle, respectively), the functional benefit of TLV was combined to a potent reduction in infarct size vs Saline and Control groups (Figure 5A). Myocardial necrosis out of the risk zone was also significantly limited by TLV as compared to Control and Saline groups (Figure 5, Panels B–D).
Figure 5.
Panel A: Infarct sizes (as percentage of the risk zone). Open and closed circles represent individual and mean infarct sizes, respectively.
Panel B: Histopathological scores of the myocardium in a territory remote to the risk zone. Lesions consisted of foci of necrosis (0=normal; 3 = frequent foci). Open circles represents individual scores.
Panel C: Histopathological appearance of the myocardium remote to the risk zone in a rabbit from the Control group.
Panel D: Normal histological appearance of the myocardium remote to the risk zone in a rabbit from the TLV group. The blue dye stains microvessels out of the risk zone.
See legend of Figure 2.
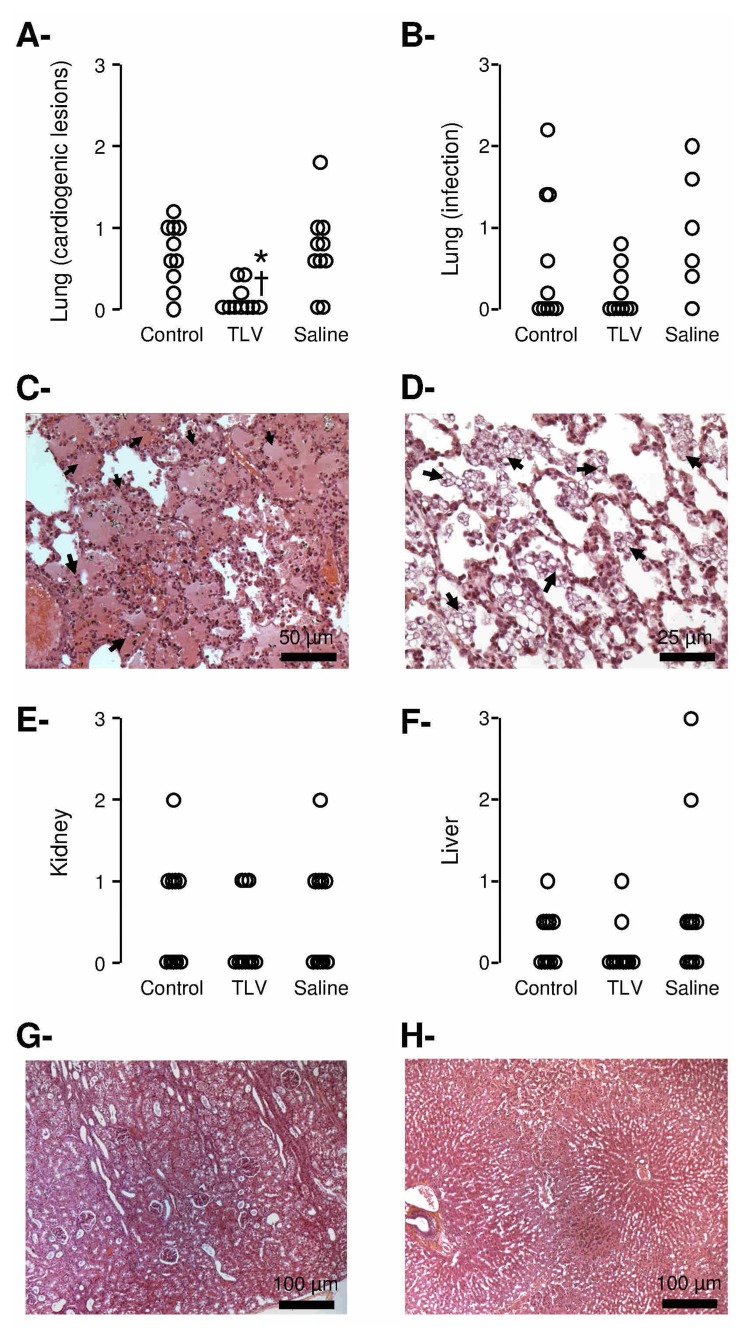
TLV limits lung cardiogenic lesions
As shown in Table IV, a metabolic acidosis was observed in all groups after cardiac arrest. One hour after coronary reperfusion, blood PO2 and pH were improved in TLV vs Control and Saline groups. At necropsy, lung examination showed reduced lung cardiogenic lesions in TLV vs Control and Saline (Figure 6, Panels A–C). Foam macrophages were identified in the TLV group in 8/10 animals (Figure 6, Panel D), probably as a consequence of some perfluorocarbons persistence. Kidney and liver dysfunctions and lesions were moderate in all groups (Table II and Figure 6, Panels E–H).
Table IV.
Blood gases and lactate blood levels.
| Baseline | Coronary reperfusion
|
||||
|---|---|---|---|---|---|
| 60 min | 180 min | Day 1 | Day 7 | ||
| Number of animals (n) | |||||
| Control | 10 | 10 | 9 | 4 | 0 |
| TLV | 10 | 10 | 10 | 9 | 8 |
| Saline | 10 | 10 | 9 | 6 | 3 |
| Lactates blood levels (mmol/l) | |||||
| Control | 3.0±0.9 | 13.5±3.1 | 11.0±3.2 | 3.3±2.9 | - |
| TLV | 3.4±0.8 | 13.7±3.3 | 12.8±3.5 | 2.3±1.1 | 3.7±1.2 |
| Saline | 3.5±1.6 | 16.1±2.1 | 15.7±2.9 * | 4.5±4.2 | 4.7±3.2 |
| Blood pH | |||||
| Control | 7.41±0.07 | 7.03±0.23 | 7.22±0.1 | 7.31±0.07 | - |
| TLV | 7.45±0.11 | 7.33±0.09 *† | 7.29±0.15 | 7.32±0.15 | 7.38±0.06 |
| Saline | 7.38±0.06 | 7.15±0.09 | 7.15±0.09 | 7.21±0.11 | 7.36±0.09 |
| Blood pCO2 (torr) | |||||
| Control | 40±8 | 41±7 | 38±4 | 57±2 | - |
| TLV | 42±10 | 28±5 * | 26±4 * | 53±10 | 43±5 |
| Saline | 42±13 | 27±5 * | 25±4 * | 57±10 | 52±10 |
| Blood pO2 (torr) | |||||
| Control | 428±129 | 205±95 | 373±136 | 57±9 | - |
| TLV | 500±170 | 456±126 *† | 354±204 | 56±18 | 78±19 |
| Saline | 526±76 | 283±121 | 305±114 | 46±10 | 67±26 |
TLV, total liquid ventilation; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling;
, p<0.05 vs corresponding Control;
, p<0.05 vs corresponding Saline.
Figure 6.
Panel A and B: Histopathological scores of cardiogenic lesions and infectious complications in the lungs, respectively. Open circles represents individual scores.
Panel C: Histopathological appearance of the lung of a rabbit in the Control group. Lesions consisted of extensive serous edema within the alveolae (arrows).
Panel D: Histopathological appearance of the lung of a rabbit in the TLV group. Foam macrophages can be seen within the alveolae (arrows).
Panels E and F: Histopathological scores of alteration of the kidney and liver, respectively. Open circles represents individual scores.
Panel G: Histopathological appearance of the kidney in the Control group. Lesions consisted of mildly dilated proximal tubules.
Panel H: Histopathological appearance of the liver of a rabbit in the Control group. Lesions consisted of systematized centrilobular congestion of the sinusoids.
See legend of Figure 2.
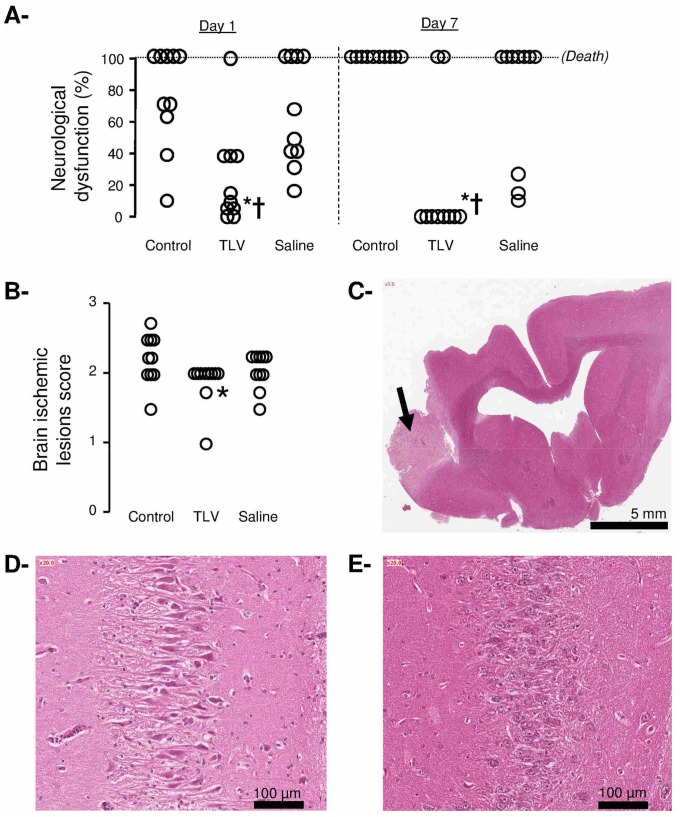
TLV limits neurological dysfunction
As illustrated in Figure 7 (Panel A), the neurological deficit score was significantly attenuated in the TLV group as compared to Control and Saline groups. This neuroprotective effect was also supported by limited brain ischemic lesions in TLV vs Control and Saline groups (Figure 7, Panels B–C). Large cerebral infarctions were observed in two rabbits from the Control and Saline groups, as well as one small and deep infarction in the TLV group.
Figure 7.
Panel A: Neurological dysfunction scores at days 1 and 7 after coronary artery occlusion and cardiac arrest in the different experimental groups. Open circles represent individual scores and the thick line represents the median value of the corresponding group.
Panel B: Histopathological scores of alteration of the brain. Open circles represents individual scores.
Panel C: Histopathological appearance of the brain in one rabbit of the Control group. Lesions consisted in a cerebral infarction (arrow).
Panel D: Histopathological appearance of the hippocampus in one rabbit of the Saline group.
Panel E: Normal histological appearance of the hippocampus in one rabbit of the TLV group.
See legend of Figure 2.
Discussion
In the present study, we demonstrate that hypothermic TLV not only attenuates neurological injury and myocardial infarct size but also prevents acute cardiovascular dysfunction and mortality after combined CAO and cardiac arrest. The favourable effect of TLV was related to cooling rapidity as a slower cooling was not similarly efficient, despite similar target temperature (32°C).
In this study, we observed a severe post-cardiac arrest syndrom (1) with an intense cardiovascular dysfunction as acute CAO and cardiac arrest were associated. In Control conditions, mortality within the first 24 h reached 60% while it was only 30% in a previous report in a similar rabbit model with 10 min of shockable cardiac arrest without CAO (12). This model is especially relevant for evaluation of acute heart failure and myocardial stunning (15) after cardiac arrest. During the early phase of global stunning (i.e. prior to coronary reperfusion), a favourable effect of ultra-fast cooling was evidenced with TLV. This was probably a direct effect of hypothermia on ventricular contractility (16, 17) as previously observed during regional stunning in rabbits submitted to focal myocardial ischemia (5). In the present study, TLV further allowed an ultimate complete recovery of left ventricular function within the first 24 h, as probably related to its potent anti-infarct effect. These functional benefits were largely related to the rapidity of cooling since conventional and slower hypothermia did not provide similar effects on cardiac output and cardiovascular mortality. These results are in line with those of Yannopoulos et al. comparing cold saline infusion to endovascular cooling in a swine model of ischemic cardiac arrest (18). Infarct size reduction was again directly related to the time to reach the target temperature. In comparison, our present study further demonstrates that the rapidity of cooling is not only critical for infarct size reduction but more importantly for prevention of cardiovascular mortality. The hemodynamic improvement afforded by TLV was however poorly predicted by blood lactate levels.
Beyond its beneficial effect on cardiovascular outcomes, this study also evidenced the neuroprotective effect of TLV through enhanced neurological recovery and decreased brain lesions. Conversely, conventional cooling was not as protective, as previously shown in a pure model of ventricular fibrillation (12). A benefit should however be evidenced with a larger number of animals as even “slow” hypothermia is known to afford a neurological benefit after cardiac arrest, although attenuated as compared to early hypothermia (7–10). This clearly contrasts with the cardioprotective effect of hypothermia that is virtually entirely lost when cooling is delayed (4–6). As an example, Knafelj et al. did not show any cardioprotective benefit of slow and externaly-induced hypothermia in survivors of ventricular fibrillation with ST-elevation acute myocardial infarction (STEMI), whereas cerebral performance category score was significantly improved (19). Clinical trials testing hypothermia in STEMI patients with no cardiac arrest also showed disapointing results when hypothermia was delayed (20) as compared to more precocious and aggressive cooling (21).
Taken together, all these findings clearly emphasized the major impact of the rapidity of cooling on outcome after combined CAO and cardiac arrest. As also observed in pigs (22, 23) and despite its invasive nature, hypothermic TLV seems to provide additional benefits to postresuscitation care. In the latter studies, some lung toxicity was however observed with the perfluorocarbon Fluorinert™ FC-77. The current development of liquid ventilators appropriate for human uses will allow confirmation of the present results for a further clinical translation (24). In the present study, we used perfluorodecalin for TLV as this coumpound was recently approved for intrapulmonary administration and bronchoalveolar lavage (Perflubronc PFD ®, Origen, Austin, Texas, USA). Perfluorodecalin is also radiolucent while the well-known perfluorooctylbromide (Perflubronc PFB ®) is opaque on X ray with potential disavantages in case of a coronary angioplasty is required in future translations. Perfluorodecalin is also a viscous liquid with a low vapour pressure as compared to other perfluorocarbons. It will be necessary to test other perfluorocarbons with a higher vapour pressure and evaporating rate (e.g., perfluorooctane). In the future, it will also be important to confirm the present results in large animals as small ones poorly mimic human inflammation states (25), that are known to be critical after cardiac arrest.
The present study has several limitations. First, a limited number of animals were euthanized for ethical reasons before the end of the follow-up (i.e., 3 Control animals). This was conducted after 48 h of follow-up when certain and rapid death was expected. Our survival analyses therefore took into account spontaneous deaths but also decision to sacrifice highly disabled animals in rare cases. Second, we did not measure coronary perfusion pressure during the cardiac massage. It would be relevant to investigate the massage efficiency.
Conclusion
In conclusion, ultra-fast cooling dramatically improved the cardiovascular outcome after cardiac arrest with underlying coronary artery occlusion. Further reports should confirm these results in larger species.
Acknowledgments
Source of Funding: This study was supported by a grant (ABYSS-R12031JJ) from the “Agence Nationale pour la Recherche” (Paris, France).
Footnotes
Conflict of Interest: R. Tissier was a recipient of a “Contrat d’Interface INSERM-ENV”. R. Tissier and A. Berdeaux are named as inventors on a patent application (13/039415).
References
- 1.Dumas F, White L, Stubbs BA, et al. Long-term prognosis following resuscitation from out of hospital cardiac arrest: role of percutaneous coronary intervention and therapeutic hypothermia. J Am Coll Cardiol. 2012;60:21–27. doi: 10.1016/j.jacc.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 3.Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 4.Hale SL, Dave RH, Kloner RA. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol. 1997;92:351–357. doi: 10.1007/BF00788947. [DOI] [PubMed] [Google Scholar]
- 5.Tissier R, Couvreur N, Ghaleh B, et al. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction. Cardiovasc Res. 2009;83:345–353. doi: 10.1093/cvr/cvp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tissier R, Hamanaka K, Kuno A, et al. Total liquid ventilation provides ultra-fast cardioprotective cooling. J Am Coll Cardiol. 2007;49:601–605. doi: 10.1016/j.jacc.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Abella BS, Zhao D, Alvarado J, et al. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 8.Kuboyama K, Safar P, Radovsky A, et al. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Nozari A, Safar P, Stezoski SW, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–2696. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- 10.Tsai MS, Barbut D, Tang W, et al. Rapid head cooling initiated coincident with cardiopulmonary resuscitation improves success of defibrillation and post-resuscitation myocardial function in a porcine model of prolonged cardiac arrest. J Am Coll Cardiol. 2008;51:1988–1990. doi: 10.1016/j.jacc.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Carroll M, Beek O. Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis. 1992;7:45–50. doi: 10.1007/BF01000440. [DOI] [PubMed] [Google Scholar]
- 12.Chenoune M, Lidouren F, Adam C, et al. Ultrafast and whole-body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits. Circulation. 2011;124:901–911. 901–907. doi: 10.1161/CIRCULATIONAHA.111.039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chenoune M, Lidouren F, Ghaleh B, et al. Rapid cooling of the heart with total liquid ventilation prevents transmural myocardial infarction following prolonged ischemia in rabbits. Resuscitation. 2010;81:359–362. doi: 10.1016/j.resuscitation.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Baker AJ, Zornow MH, Grafe MR, et al. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. 1991;22:666–673. doi: 10.1161/01.str.22.5.666. [DOI] [PubMed] [Google Scholar]
- 15.Kern KB, Hilwig RW, Rhee KH, et al. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzl M, Steendijk P, Huber S, et al. The induction of mild hypothermia improves systolic function of the resuscitated porcine heart at no further sympathetic activation. Acta Physiol (Oxf) 2011;203:409–418. doi: 10.1111/j.1748-1716.2011.02332.x. [DOI] [PubMed] [Google Scholar]
- 17.Gotberg M, van der Pals J, Olivecrona GK, et al. Mild hypothermia reduces acute mortality and improves hemodynamic outcome in a cardiogenic shock pig model. Resuscitation. 2010;81:1190–1196. doi: 10.1016/j.resuscitation.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Yannopoulos D, Zviman M, Castro V, et al. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation. 2009;120:1426–1435. doi: 10.1161/CIRCULATIONAHA.109.848424. [DOI] [PubMed] [Google Scholar]
- 19.Knafelj R, Radsel P, Ploj T, et al. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation. 2007;74:227–234. doi: 10.1016/j.resuscitation.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Grines CL on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at the 16th annual Transcatheter Cardiovascular Therapeutics; Washnington DC. September 2004. [Google Scholar]; O’Neill WW, Dixon SR, Grines CL. The year in interventional cardiology. J Am Coll Cardiol. 2005;45:1117–1134. doi: 10.1016/j.jacc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Gotberg M, Olivecrona GK, Koul S, et al. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv. 2010;3:400–407. doi: 10.1161/CIRCINTERVENTIONS.110.957902. [DOI] [PubMed] [Google Scholar]
- 22.Riter HG, Brooks LA, Pretorius AM, et al. Intra-arrest hypothermia: both cold liquid ventilation with perfluorocarbons and cold intravenous saline rapidly achieve hypothermia, but only cold liquid ventilation improves resumption of spontaneous circulation. Resuscitation. 2009;80:561–566. doi: 10.1016/j.resuscitation.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staffey KS, Dendi R, Brooks LA, et al. Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation. 2008;78:77–84. doi: 10.1016/j.resuscitation.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avoine O, Bosse D, Beaudry B, et al. Total liquid ventilation efficacy in an ovine model of severe meconium aspiration syndrome. Crit Care Med. 2011;39:1097–1103. doi: 10.1097/CCM.0b013e31820ead1a. [DOI] [PubMed] [Google Scholar]
- 25.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]