Abstract
Objective
Review the outcome of patients with complex fractures around the knee treated with megaprosthesis.
Method
Retrospective observational study of 10 patients was undertaken.
Results
Six patients were treated with a distal femoral endoprosthesis (DEFPR) and four with an augmented rotating hinge knee replacement (RHK). The mean post-operative Toronto Extremity Salvage Score (TESS) was 62.5 for the whole cohort (RHK group 79.3, compared with 49.2 in the DFEPR group (p = 0.038), peri-prosthetic fracture group was 46.3, compared with 75.6 in native knee fracture group (p = 0.04)).
Conclusion
A megaprosthesis is a viable option in complex fractures around the knee.
Keywords: Complex, Knee, Trauma, Megaprosthesis
1. Introduction
Limb salvage surgery has become the standard for local control of aggressive bone and soft tissue tumours arising around the knee.1 As such, tumour units have been able to combine the advances in metallurgical techniques with the need to replace large bone segments after resection for oncological indications.2 Modular megaprosthesis are defined as special bone and joint prostheses, which can bridge and compensate for large bone defects caused by loss of bone stock.3
An accurate incidence of peri-articular fractures of the knee is difficult to obtain as it varies with both geographic and population demographics.4 Court-Brown and Caesar5 performed a review of nearly 6000 fractures, reporting the incidence of proximal tibial fractures as 13.3 per 100,000 adult population, with an incidence of 4.5 per 100,000 for distal femoral fractures. The majority of these fractures occur in older patients, with 86% of distal femoral fractures occurring in patients more that 70 years of age and 24% of proximal tibial fractures occurring in patients more than 65 years old.5, 6
The number of total knee arthroplasties performed continues to rise annually and it would be expected that complications, which include periprosthetic fractures, will also therefore become more commonplace.7 This type of injury can have potentially devastating complications to the patient and poses significant challenges to the surgical team. The management of these often complex fractures depends on displacement at the fracture site, bone quality, size of the fragments and condition of implants.7 The over riding aim is to maintain lower limb alignment whilst providing a stable reconstruction to allow the patient an early return to ambulation as possible.
Some reports exist detailing the use of megaprostheses in non-oncological musculoskeletal conditions,3 where bone stock has been severely compromised. We present the outcome of patients managed at our specialist, tertiary orthopaedic oncology unit referred with traumatic fractures involving a native or prosthetic knee.
2. Method
We conducted a retrospective review of all patients managed at our tertiary orthopaedic oncology unit with knee trauma. All patients had been referred to our unit between 2009 and 2012 for a specialist opinion of their complex fractures involving the knee.
For each patient, demographic data were collected along with pain scores out of 10 pre- and post-definitive surgeries (10 being maximally severe pain, 0 no pain). All patients completed a Toronto Extremity Salvage Score (TESS) 6–12 months after their definitive operative intervention at our institution.8, 9 The TESS is based on the definitions of disability, impairment and handicap as documented by the World Health Organisation.10 It is a well-recognised tool assessing functional outcomes following surgery for an extremity sarcoma and has been tested for validity and reliability.9, 11 It consists of a series of questions, with the maximum score achievable being 100 signifying no impairment to function. TESS is commonly used at our institution to monitor the post-operative functional improvement in our musculoskeletal oncology patients.
Specific to the knee injury, the AO12 system was used to classify fractures around a native joint. With regards to peri-prosthetic fractures around a total knee replacement, the Rorabeck13 system was used to classify fractures involving the femoral component and the Felix14 system was used to classify fractures involving the tibial component.
Simple summary statistics were collated using SPSS v 18.0 software (IBM Corp., Armonk, New York). Continuous data were compared using an unpaired t-test, with an alpha value of 0.05 considered statistically significant.
3. Results
10 patients were identified. All had closed injuries. 5 were male. All underwent single-stage operative intervention utilising a megaprosthesis to definitively manage their complex knee fractures. The mean age at definitive surgery was 70.2 years (48–94 years). Six patients were treated with a distal femoral endoprosthesis (DFEPR, Stanmore Implants, Elstree, United Kingdom), with four requiring a rotating hinge knee replacement (RHK) (Biomet Orthopedics, Warsaw, IN, USA). The mean post-operative TESS was 62.5 for the whole cohort. The mean post-operative TESS in the RHK group was 79.3, compared with a mean TESS of 49.2 in the DFEPR group (p = 0.038). The mean post-operative TESS in patients with a peri-prosthetic fracture was 46.3, compared with a mean TESS of 75.6 in those sustaining a fracture involving a native knee (p = 0.04). The mean pre-definitive surgery pain score was 7.8/10, while the mean post-definitive surgery pain score was 2.0/10. All patients reported improvement in their pain.
The mean follow-up was 3 years. At last follow-up, all patients were mobile, either with or without walking aids, and no cases of deep infection or loosening have been reported. One patient (case 9) died prior to collection of a TESS score from causes unrelated to their definitive surgery. Table 1 summarises the results.
Table 1.
Summary of results.
| Case | Age | Indication | Classification | Intervention | TESS |
|---|---|---|---|---|---|
| 1 | 74 | Multiple osteoporotic fractures | AO 41 C3 | RHK | 85 |
| 2 | 65 | Tibial fracture | AO 41 C3 | RHK | 90 |
| 3 | 94 | Periprosthetic fracture | Felix II | DFEPR | 28 |
| 4 | 68 | Periprosthetic fracture | Rorabeck II | DFEPR | 25 |
| 5 | 53 | Fractured distal femur in RTA and non union | AO 33 C3 | DFEPR | 70 |
| 6 | 63 | Proximal tibial fracture | AO 41 C3 | RHK | 71 |
| 7 | 65 | Periprosthetic fracture | Rorabeck III | RHK | 71 |
| 8 | 48 | RTA | AO 33 C3 | DFEPR | 62 |
| 9 | 89 | Osteoporotic periprosthetic fracture | Rorabeck III | DFEPR | No score |
| 10 | 83 | Periprosthetic fracture | Rorabeck III | DFEPR | 61 |
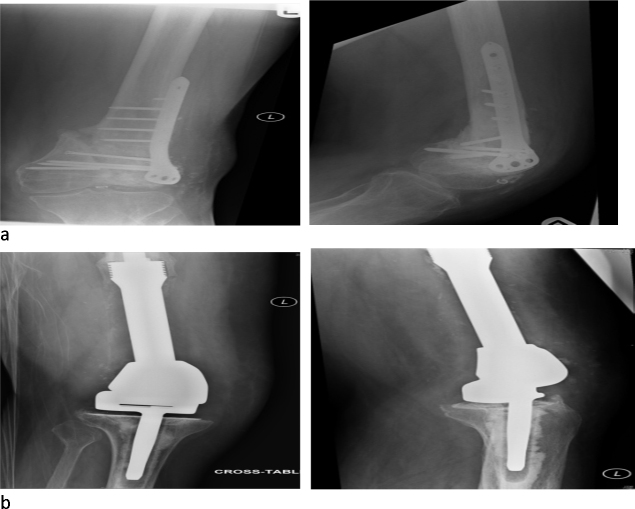
Case 5 was a 53-year-old male involved in a high speed motor cycle accident (Fig. 1a and b). He sustained a closed, isolated injury to the distal femur (AO 33 C3) that was treated by open reduction and internal fixation (ORIF). He was referred to our unit 6 months after the initial surgery with a painful non-union. He underwent a DFEPR, and at last follow-up was independently mobile.
Fig. 1.
Case 5: (a) pre-DFEPR and (b) post-DFEPR.
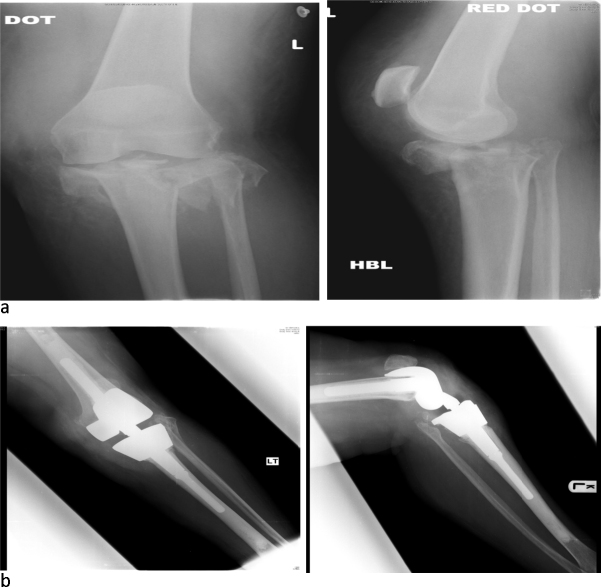
Case 6 was a 63-year-old female referred directly to us from a local hospital with a severely comminuted proximal tibial fracture (AO 41 C3). The referring trauma unit did not feel reconstruction using ORIF was possible. She underwent salvage reconstruction using a RHK (Fig. 2a and b). At last follow-up, she was independently mobile.
Fig. 2.
Case 6: (a) pre-RHK and (b) post-RHK.
4. Discussion
This study shows that megaprostheses can be employed to negate the difficulties in limb reconstruction in patients presenting with complex fractures around the knee. The patients presented are an extreme, highly specialised, heterogeneous group, who were faced with a drastic situation of having to consider unpredictable and undesirable operative interventions such as ORIF, arthrodesis or amputation. Our tertiary orthopaedic oncology unit has significant expertise in limb salvage and reconstruction using megaprostheses in the field of musculoskeletal malignancy. We utilised the same techniques we would use in the management of bone defects following tumour resection in the salvage procedures presented in this study.
Numerous reconstructive strategies exist to treat large bone defects including allograft,15, 16, 17 biotechnologies in mono or polytherapy, standard arthroplasty and, lastly, megaprosthesis.2 Structural allografts have a complication rate of 23–55%18, 19, 20 and include allograft resorption, non-union, spontaneous fracture and infection.21, 22 In addition to the complications reported with the use of allografts, one major disadvantage is that immediate full weight bearing is usually not possible, as the bone requires a period of time to consolidate.23 This period of restricted mobility may be associated with medical complications,24 particularly in those patients with advanced age.
The necessity to resect large bone segments in the field of orthopaedic oncology has driven the development of megaprosthesis. They provide the surgeon with the ability to replace significant bone and joint defects whilst, simultaneously, providing the patient with a functional limb that may have otherwise been amputated. Furthermore, a patient with a more functional limb has an improved quality of life.25, 26
Megaprostheses are relatively easy to use and the systems can be highly modular allowing various resections to fit as many patients as possible.3 Indeed, custom-made implants are also available. Megaprostheses provide a consistently more predictable outcome.1 Even in patients with a poor prognosis, they have a role in optimising quality of life, providing pain relief, and maintaining an intact body image.27 Importantly, arthrodesis and amputation are consistently reported to have poor patient outcomes.28, 29
Although we do not report any infections in our study, high rates of periprosthetic infection are reported in the literature.30 When used in oncological cases, distal femoral replacement has a reported infection rate of 20%,23 whilst infection around proximal tibial replacements is as high as 50%.23 This may be due to a relatively poor soft-tissue envelope covering the proximal tibia, despite plastic surgical coverage with a gastrocnemius rotation flap. The use of silver-coated megaprosthesis has been reported to reduce the rate of periprosthetic infection.31, 32 We did not use these implants in this study.
Due to the relatively short follow-up period we present, no evidence of aseptic loosening has been noted. Aseptic loosening in oncological cases is reported to occur in 24% of distal femoral replacements at 10 years.33 With longer follow-up, we would expect the rate of aseptic loosening to increase in our cohort of patients. Fixing the shaft using a hydroxyapatite-coated collar appears to reduce the loosening rate,33 a technique we used in all of the DFEPRs.
The TESS outcomes we present in this study are similar to those of a previous study from our unit of megaprosthesis around the knee in orthopaedic oncology patients, which revealed a mean TESS of 72.34 It must be borne in mind, however, that bone sarcoma patients differ significantly to the trauma cohort we present. Patients with primary bone tumours are often young and otherwise fit, and have good bone quality. This contrasts starkly with the more frail elderly patients seen in our study. The expectations of these two groups are likely to be different with those with malignant tumours being primarily concerned with disease-free survival. In general, all patients cope well with activities of daily living, such as, standing and walking, washing and dressing, and light shopping. Unsurprisingly, most patients struggle with heavy activities, such as, gardening and sports.
The present study confirms that a periprosthetic fracture is associated with a poorer functional outcome when compared to a fracture involving a native knee joint (post-operative TESS = 46 vs 75.6 (p = 0.04)). However, in these complex cases, revision surgery using a megaprosthesis is the only option for preserving the extremity whilst allowing mobilisation and full weight-bearing as early as possible.2
The study has several limitations. Due to the highly specialised and rare indications, the number of patients is small. Only a retrospective analysis of the group was possible, and no pre-operative TESS was measured. Other scoring systems could have been employed; however, because our unit is primarily a tertiary orthopaedic oncology unit and, due to our familiarity with the scoring system, we deemed the TESS to be the most appropriate tool for comparison of functional outcomes.
5. Conclusion
The use of a megaprosthesis is a viable option in cases, where bone stock at the fracture site is so severely compromised that traditional osteosynthesis or revision arthroplasty techniques would not provide a stable, durable implant to allow early mobilisation. Patients with traumatic injuries requiring a megaprosthesis have a significantly disabling disease. They should be treated in specialist units familiar with the technology used, and the rehabilitation required, so they can achieve, as best as possible, restitutio ad integrum.
Conflicts of interest
The authors have none to declare.
References
- 1.Gebhart M., Shumelinsky F. Management of periprosthetic fractures in patients treated with a megaprothesis for malignant bone tumours around the knee. Acta Orthop Belg. 2012;78:558–563. [PubMed] [Google Scholar]
- 2.Calori G.M., Colombo M., Ripamonti C. Megaprothesis in large bone defects: opportunity or chimaera? Injury. 2013;45:388–393. doi: 10.1016/j.injury.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Lundh F., Sayed-Noor A.S. Megaprosthetic reconstruction for periprosthetic or highly comminuted fractures of the hip and knee. Eur J Orthop Surg Traumatol. 2014;24:553–557. doi: 10.1007/s00590-013-1237-7. [DOI] [PubMed] [Google Scholar]
- 4.Mallina R., Kanakaris N.K., Giannoudis P.V. Peri-articular fractures of the knee: an update on current issues. Knee. 2010;17:181–186. doi: 10.1016/j.knee.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Court-Brown C.M., Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37:691–697. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 6.Nourissat G., Hoffman E., Hémon C. Total knee arthroplasty for recent severe fracture of the proximal tibial epiphysis in the elderly subject. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:242–247. doi: 10.1016/s0035-1040(06)75731-2. [in French] [DOI] [PubMed] [Google Scholar]
- 7.Sarmah S.S., Patel S., Reading G., El Husseiny M., Douglas S., Haddad F.S. Ann R Coll Surg Engl. 2012;94:302–307. doi: 10.1308/10.1308/003588412X13171221592537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis A.M., Bell R.S., Badley E.M., Yoshida K., Williams J.I. Evaluating functional outcome in patients with lower extremity sarcoma. Clin Orthop Relat Res. 1999;358:90–100. [PubMed] [Google Scholar]
- 9.Davis A.M., Wright J.G., Williams J.I., Bombardier C., Griffin A., Bell R.S. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva, Switzerland: 1990. International Classification of Impairments, Disabilities and Handicaps. [Google Scholar]
- 11.Schreiber D., Bell R.S., Wunder J.S. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcom. Qual Life Res. 2006;15:1439–1446. doi: 10.1007/s11136-006-0001-4. [DOI] [PubMed] [Google Scholar]
- 12.Müller M.E., Nazarian S., Koch P. 1st ed. Springer-Verlag; Berlin, Heidelberg, New York: 1990. The Comprehensive Classification of Fractures of Long Bones. [Google Scholar]
- 13.Rorabeck C.H., Angliss R.D., Lewis P.L. Fractures of the femur, tibia, and patella after total knee arthroplasty: decision making and principles of management. Instr Course Lect. 1998;47:449–460. 47449. [PubMed] [Google Scholar]
- 14.Felix N.A., Stuart M.J., Hanssen A.D. Periprosthetic fractures of the tibia associated with total knee arthroplasty. Clin Orthop Relat Res. 1997;345:113–124. [PubMed] [Google Scholar]
- 15.Calori G.M., Giannoudis P.V. Enhancement of fracture healing with the diamond concept: the role of the biological chamber. Injury. 2011;42:1191–1193. doi: 10.1016/j.injury.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Calori G.M., Mazza E., Colombo M., Ripamonti C., Tagliabue L. Treatment of long bone non-unions with polytherapy: indications and clinical results. Injury. 2011;42:587–590. doi: 10.1016/j.injury.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 17.Calori G.M., Colombo M., Ripamonti C., Bucci M., Fadigati P., Mazza E. Polytherapy in bone regeneration: clinical applications and preliminary considerations. Int J Immunopathol Pharmacol. 2011;24(Suppl. 2):85–89. doi: 10.1177/03946320110241S216. [DOI] [PubMed] [Google Scholar]
- 18.Bauman R.D., Lewallen D.G., Hanssen A.D. Limitations of structural allograft in revision total knee arthroplasty. Clin Orthop Relat Res. 2009;467:818–824. doi: 10.1007/s11999-008-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clatworthy M.G., Balance J., Brick G.W., Chandler H.P., Gross A.E. The use of structural allograft for uncontained defects in revision total knee arthroplasy. A minimum five-year review. J Bone Joint Surg Am. 2001;83A:404–411. doi: 10.2106/00004623-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Mnaymneh W., Emerson R.H., Borja F., Head H.C., Malinin T.I. Massive allografts in salvage revisions of failed total knee arthroplasties. Clin Orthop Relat Res. 1990;260:144–153. [PubMed] [Google Scholar]
- 21.Mow C.S., Wiedel J.D. Structural allografting in revision total knee arthroplasty. J Arthroplasy. 1996;11:235–241. doi: 10.1016/s0883-5403(96)80072-6. [DOI] [PubMed] [Google Scholar]
- 22.Wunder J.S., Leitch K., Griffin A.M., Davis A.M., Bell R.S. Comparison of two methods of reconstruction for primary malignant tumours at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99. doi: 10.1002/jso.1076. [DOI] [PubMed] [Google Scholar]
- 23.Höll S., Schlomberg A., Gosheger G. Distal femur and proximal tibia replacement with megaprothesis in revision knee arthroplasty: a limb-saving procedure. Knee Surg Sports Traumatol Arthrosc. 2012;20:2513–2518. doi: 10.1007/s00167-012-1945-2. [DOI] [PubMed] [Google Scholar]
- 24.Zuckermann J.D., Skovron M.L., Koval K.J., Aharonoff G., Frankel V.H. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77:1551–1556. doi: 10.2106/00004623-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hillmann A., Hoffmann C., Gosheger G., Krakau H., Winkelmann W. Malignant tumour of the distal part of the femur or the proximal of the tibia: endoprosthetic replacement or rotationplasty. Functional outcome and quality-of life measurements. J Bone Joint Surg Am. 1999;81:462–468. doi: 10.2106/00004623-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Kawai A., Muschler G.F., Lane J.M., Otis J.C., Healey J.H. Prosthetic knee replacement after resection of a malignant tumour of the distal part of the femur. Medium to long-term results. J Bone Joint Surg Am. 1998;80:636–647. doi: 10.2106/00004623-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Zahlten-Hinguranage A., Bernd L., Sabo D. Amputation or limb salvage? Assessing quality of life after tumor operations of the lower extremity. Orthopade. 2003;32:1020–1027. doi: 10.1007/s00132-003-0548-5. [DOI] [PubMed] [Google Scholar]
- 28.Harris I.E., Leff A.R., Gitelis S., Simon M.A. Function after amputation, arthrodesis, or arthroplasty for tumors about the knee. J Bone Joint Surg Am. 1990;72:1477–1485. [PubMed] [Google Scholar]
- 29.Rougraff B.T., Simon M.A., Ishii T., Motojima S., Tokuhashi Y., Ryu J. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Hardes J., Gebert C., Schwappach A. Characteristics and outcome of infections associated with tumor endoprosthesis. Arch Orthop Trauma Surg. 2006;126:289–296. doi: 10.1007/s00402-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 31.Gosheger G., Goetze C., Hardes J., Joosten U., Winkelmann W., von Eiff C. The influence of the alloy of megaprothesis on infection rate. J Arthroplast. 2008;23:916–920. doi: 10.1016/j.arth.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Hardes J., von Eiff C., Streitbuerger A. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 33.Myers G.J., Abudu A.T., Carter S.R., Tillman R.M., Grimer R.J. Endoprosthetic replacement of the distal femurs for bone tumours. Long-term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 34.Gendall E., Rathore R. British Sarcoma Group Annual Conference. 2008. Functional outcomes following surgery for bone sarcomas. [Google Scholar]