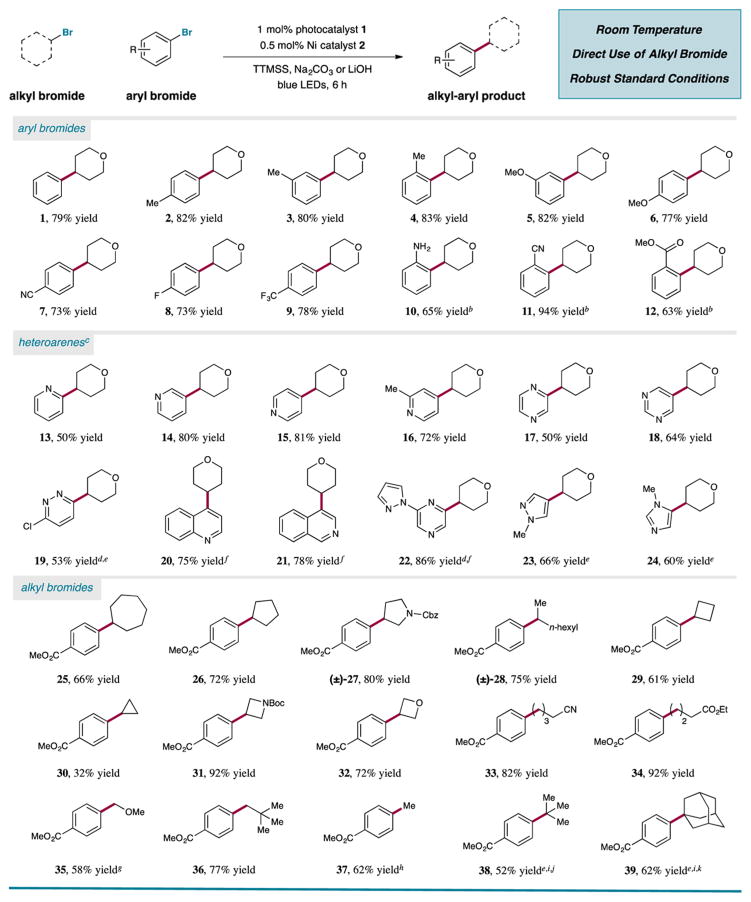
Table 2.
Photoredox-Enabled Csp3–Csp2 Coupling from Alkyl and Aryl Halide Precursors: Comprehensive Scope of Productsa
All yields are isolated yields using photocatalyst 1 (1 mol %), NiCl2·dtbbpy (0.5 mol %), aryl halide (0.5 mmol), alkyl halide (0.75 mmol), TTMSS
(0.5 mmol), and Na2CO3 (2 equiv).
12 h
LiOH as base.
Aryl chloride.
10 mol % 2.
5 mol % 2.
MOMCl.
MeOTs and LiBr.
Dioxane as solvent.
48 h.
24 h.