Figure 1.
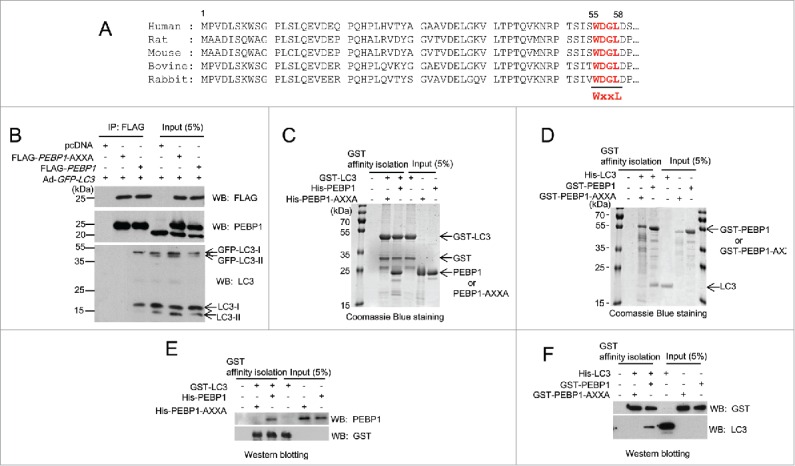
PEBP1 binds directly to LC3 in cells and in vitro. (A) Alignment of PEBP1 amino acid sequences across various species as shown. The N-terminal 60 amino acids of the PEBP1 genes were aligned. The conserved LC3-interacting region (WXXL) of PEBP1 (55th-58th amino acids) is represented in bold. (B) HeLa cells were transiently transfected with plasmids expressing FLAG-PEBP1 or FLAG-PEBP1-AXXA. The pcDNA plasmid was transfected as a negative control. The mutant PEBP1-AXXA protein has alanine residues substituted for Trp55 and Leu58. After 24 h, HeLa cells were infected with recombinant adenoviruses expressing GFP-LC3 (Ad-GFP-LC3). FLAG-tagged PEBP1 proteins (1 mg total protein) were immunoprecipitated with beads conjugated to anti-FLAG M2 antibodies. Bound LC3 proteins were analyzed by western blot using anti-LC3 antibodies. Total PEBP1 proteins (5% input) in whole cell extracts were assessed by western blot using anti- PEBP1 antibody. (C, E) GST-LC3 affinity isolation with His6-tagged PEBP1 in vitro. GST-LC3 protein immobilized to glutathione beads was incubated with either purified His6-PEBP1 or His6-PEBP1-AXXA protein for 1 h at 4°C. Following extensive washing, the bound proteins were subjected to 10% SDS-PAGE and visualized by Coomassie Blue G-250 staining (C) or western blot using anti-PEBP1 or anti-GST antibodies (E). Total protein used for GST affinity isolation is represented as input (5%). (D, F) GST-PEBP1 affinity isolation with His6-tagged LC3 in vitro. GST-PEBP1 or GST-PEBP1-AXXA proteins immobilized to glutathione beads were incubated with purified His6-LC3 proteins for 1 h at 4°C. After washing, bound proteins were analyzed by 10% SDS-PAGE and visualized by Coomassie Blue G-250 staining (D) or western blot using anti-GST or anti-LC3 antibodies (F).
