Figure 7.
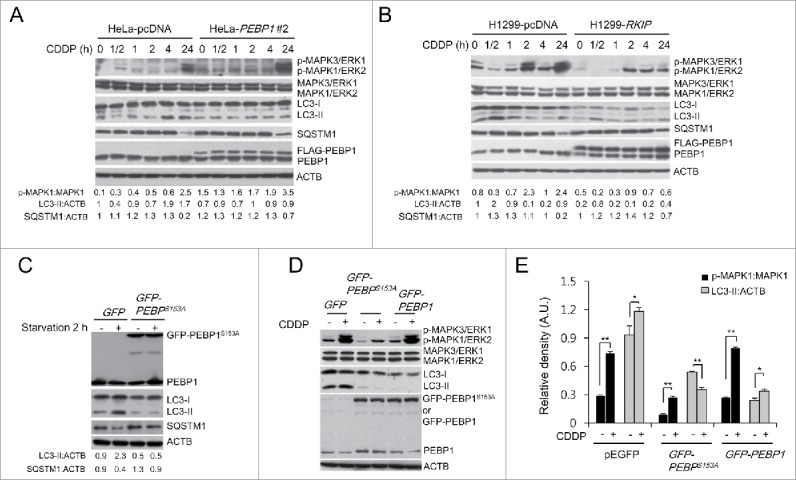
PEBP1 inhibits autophagy in a MAPK/ERK-independent manner. (A, B) HeLa (A) or H1299 cells (B) stably selected with control pcDNA vector or FLAG-PEBP1-expressing plasmid were treated with 20 μM of cisplatin (CDDP) for the indicated times. Protein and phosphorylation levels were examined by western blot using specific antibodies and quantified. ACTB was used as a loading control. Numbers below each lane indicate the relative signal intensity ratio of LC3-II:ACTB, SQSTM1:ACTB, and p-MAPK1/ERK2:MAPK1, as calculated from 3 independent experiments. (C) Effect of PEBP1 mutant proteins (PEBP1S153A) abrogating PEBP1 dissociation from RAF1 on starvation-induced autophagy. HeLa cells were transiently transfected with plasmids encoding mutant PEBP1 (GFP-PEBP1S153A). After 24 h, cells were starved for 2 h, and proteins were then analyzed by western blot and quantified. Numbers below each lane indicate the intensity ratio between LC3-II, or SQSTM1 and ACTB, calculated from 3 independent experiments. (D) Effect of PEBP1 mutant proteins (PEBP1S153A) on CDDP-induced autophagy. HeLa cells transiently expressing GFP-PEBP1S153A, GFP-PEBP1 or GFP were treated with 20 μM CDDP for 24 h, and total cell extracts were analyzed by western blotting using phospho-MAPK/ERK, MAPK/ERK, LC3, and PEBP1 antibodies. (E) The level of protein and phosphorylation was quantified using the NIH ImageJ software (NIH version 1.49). Data represent the mean (± SD) of 3 independent experiments (*p < 0.05, **p < 0.01, compared with untreated control). A.U., arbitrary units.
