Figure 7.
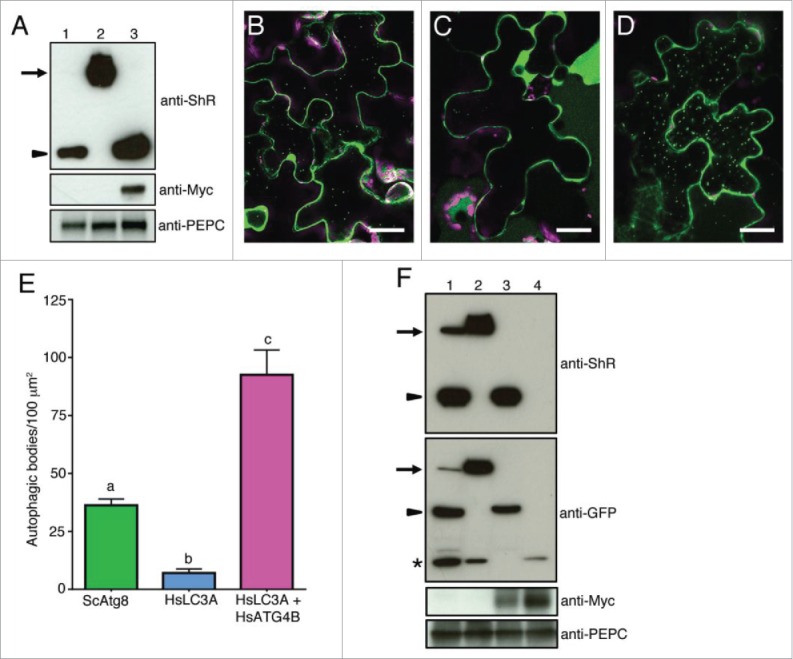
Nicotiana benthamiana plant ATG4 efficiently process yeast Atg8 but weakly process human LC3A. (A) C-ScAtg8-ShR (lane 1), C-HsLC3A-ShR (lane 2), and C-HsLC3A-ShR with HsATG4B (lane 3) were expressed in N. benthamiana plant leaves and cleavage byproduct (ShR) was detected with anti-ShR antibodies (top panel). The cleaved ShR byproduct was observed in C-ScAtg8-ShR-expressing tissue (lane 1) but not in C-HsLC3A-ShR (lane 2). Coexpression of C-HsLC3A-ShR with HsATG4B resulted in accumulation of the cleaved ShR byproduct (lane 3). Arrow and arrowhead indicate full-length ATG8 sensors and cleaved byproducts, respectively. Anti-MYC was used to detect the HsATG4B input (middle panel, lane 3). Anti-PEPC was used for input loading control (bottom panel). (B to D) C-ScAtg8-ShR sensor was processed by endogenous N. benthamiana ATG4s and the mature form of C-ScAtg8 was incorporated into autophagic bodies in the vacuole (B). Coexpression of C-HsLC3A-ShR with HsATG4B showed enhanced accumulation of autophagic bodies in the vacuole (D) compared to the expression of C-HsLC3A-ShR alone (C) in N. benthamiana leaves. Scale bar: 20 µm. (E) Quantification of autophagic bodies observed in (B to D). One-way ANOVA test indicates a statistically different number of autophagic bodies accumulated in the vacuole of cells expressing ScATG8 alone and HsLC3A with HsATG4B compared to HsLC3A alone. Lowercase letters indicate statistical differences (P < 0 .0001). (F) C-ScAtg8-ShR (lane 1), C-HsLC3A-ShR (lane 2), C-HsLC3A-ShR with HsATG4B (lane 3), and Citrine alone with HsATG4B (lane 4) were expressed in N. benthamiana plant leaves. After dark-induced autophagy, the isolated proteins were separated on SDS-PAGE and probed with anti-ShR antibodies (top panel). C-HsLC3A-ShR synthetic substrate (top panel, lane 2) could not be processed by endogenous N. benthamiana ATG4s compared to ScAtg8 (top panel, lane 1) in the dark-induced autophagy condition. The HsLC3A was efficiently processed when coexpressed with HsATG4B (top panel, lane 3). Anti-GFP antibody was used to detect free Citrine fluorescent protein and mature processed C-ATG8A or -LC3A (second panel). Anti-MYC was used to detect the HsATG4B input (third panel). Anti-PEPC was used for input loading control (bottom panel). Arrows and arrowheads indicate full-length synthetic sensors and cleaved byproducts, respectively. Asterisk represents free Citrine.
