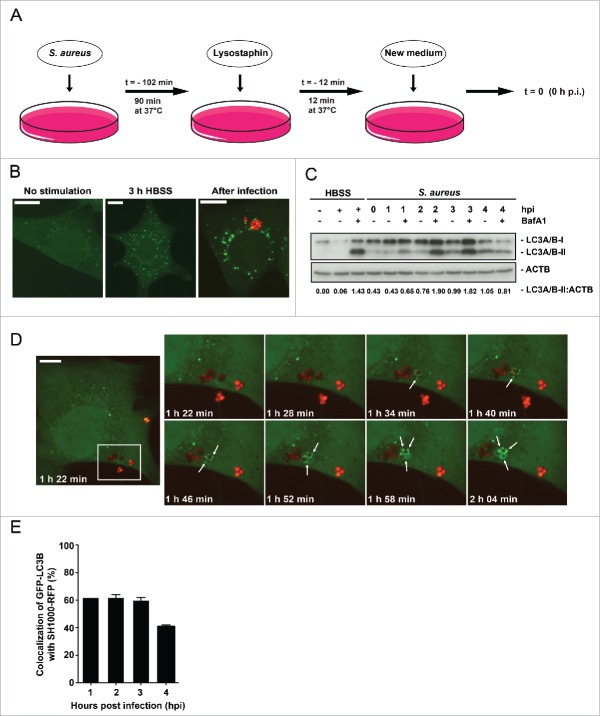
Figure 1.
Enclosure of intracellular S. aureus into GFP-LC3B-positive compartments. (A) Schematic representation of our experimental design. Eukaryotic cells were incubated with S. aureus for 90 min at 37°C. Afterwards, all extracellular bacteria were killed by the addition of lysostaphin. After 12 min incubation at 37°C the media was changed and recording of the experimental process started with time point 0 h postinfection (hpi). (B) NIH/3T3 cells stably expressing GFP-LC3B were either starved 3 h in HBSS, left untreated (no stimulation) or infected with RFP-expressing S. aureus for 1 hpi. Subsequently, cells were fixed and analyzed by confocal microscopy for GFP-LC3B expression as well as RFP-expressing S. aureus. Scale bars: 12 µm. (C) Wild-type MEF cells were infected with wild-type SH1000 for the indicated time periods. Uninfected cells and cells starved for 3 h in HBSS (with and without 100 nM Baf A1 for the last 2 h of culturing to prevent LC3-II degradation) served as negative and as positive controls, respectively. Cellular lysates were prepared and analyzed by immunoblotting using indicated antibodies. Normalization of ACTB/actin in comparison to LC3A/B-II expression was analyzed by ImageJ. (D) NIH/3T3 GFP-LC3B cells were infected with SH1000-RFP and recorded via live-cell imaging (recording 3 time points/min), shown are 8 time points. Arrowheads show the appearance of LC3B-positive puncta to intracellular S. aureus. Scale bars: 11 µm. (E) Quantification of the experiment shown in (D) for 1, 2, 3 and 4 hpi. The percentage of intracellular S. aureus colocalizing with GFP-LC3B is shown. 50 cells per time point were analyzed. Data are represented as mean ± SEM of 2 independent experiments.