Figure 1.
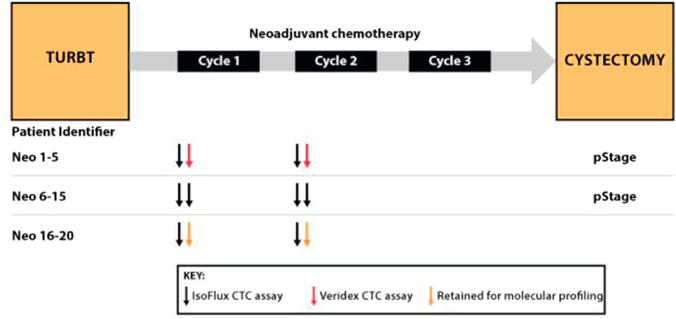
Outline of neoadjuvant study endpoints. At time of transurethral resection of bladder tumor (TURBT) 20 patients with neoadjuvant bladder cancer were enrolled. In all patients 2 tubes of blood were drawn before first neoadjuvant chemotherapy cycle and 2 were drawn after first cycle approximately 1 month later. First 5 patients had 1 blood tube drawn and tested on IsoFlux System and second blood tube tested on CellSearch assay. Next 10 patients had 2 tubes analyzed on IsoFlux System to assess repeatability. Remaining 6 patients had 2 tubes processed on IsoFlux System but 1 was retained for molecular profiling. Pathological stage was available in first 17 patients.
