Abstract
Introduction:
A significant minority of patients continue to smoke after a cancer diagnosis. Cancer patients who smoke experience stigma that can negatively impact health outcomes. We explored publicly shared perspectives about cancer patients who continued to smoke post-diagnosis.
Methods:
An online news article, published in January 2012, summarized the findings of smoking prevalence among patients with lung cancer and colorectal cancer enrolled in the Cancer Care Outcomes Research and Surveillance Consortium trial. In response, written comments were posted on the articles’ public discussion board. Applying principles of grounded theory, we conducted a document analysis and established a conceptual framework to develop a model by which to explain factors underlying stigmatic and sympathetic attitudes toward cancer survivors who continue to smoke.
Results:
Personal experiences with cancer, smoking, and statistical literacy were found to influence beliefs about cancer and smoking, which in turn influenced stigmatic or sympathetic attitudes. More sympathetic attitudes were expressed by individuals who had personal experiences with smoking, believed cancer is multicausal, identified smoking as an addiction, or considered extrinsic factors responsible for smoking. Individuals who did not have personal experiences with cancer or smoking, had low statistical literacy, believed that smoking necessarily and directly causes cancer, and focused on intrinsic responsibilities for smoking tended to express more stigmatic attitudes.
Conclusions:
The current findings raise awareness and provide insight into stigma against cancer survivors who smoke and can help inform strategies for reducing stigma against this vulnerable group.
Implications:
This study helps raise awareness of stigma toward cancer patients who smoke and provides insight into the processes that may influence stigmatic as compared to sympathetic attitudes toward these patients. Results suggest that population-based strategies to educate the public regarding the nature of nicotine addiction, difficulty of quitting, and benefits of quitting for cancer patients may be useful for reducing stigma against cancer patients with a smoking history.
Introduction
Cigarette smoking remains the leading cause of preventable death and disability in the United States, accounting for 1 in 5 deaths and nearly $170 billion in health care costs each year.1,2 Smoking prevalence rates have remained relatively stable over the past 20 years with 16.8% of adults in the United States being current regular cigarette smokers.3,4 Smoking is well-known to damage nearly every organ of the body and cause a range of health problems including several different types of cancer (eg, lung cancer and kidney cancer).5 In 2014, almost 176 000 of the estimated 585 720 cancer deaths were caused by tobacco use.6 Lung cancer is the leading cause of cancer death in the United States, and cigarette smoking is responsible for 87% of lung cancers.6 Given these negative health effects, many smokers are motivated to quit smoking; nearly 70% report a desire to quit in any given year and, of those, approximately half make a quit attempt.7 Unfortunately, most smokers who attempt to quit are not successful, with only 6% maintaining smoking cessation for 6 months or more.7
Regular cigarette smoking reflects a strong physiological and psychological addiction to nicotine. As such, many people continue to smoke even after being diagnosed with a smoking-related disease, such as cancer. In our previous study of 2456 lung cancer and 3063 colorectal cancer patients enrolled in the Cancer Care Outcomes Research and Surveillance Consortium trial between 2003 and 2005, a significant minority (14% and 9%, respectively) were smoking at 5 months post-diagnosis.8 This is of concern, because continuing to smoke following a cancer diagnosis is associated with decreased survival time, increased complications of cancer treatments, and increased risk of second primary tumors.9–12 Although most cancer patients who continue to smoke post-diagnosis report a desire to quit, patients are often unsuccessful because they are highly nicotine dependent13 and do not receive proper behavioral and pharmacological assistance.14–17 Indeed, while undergoing cancer treatment, smoking cessation is infrequently addressed; one study showed that oncologists offered smoking cessation counseling to only 25% of their smoking patients.18 For smokers who are able to quit during active treatment, nearly 50% relapse to smoking after treatment completion.19,20
Stigma may contribute to a cancer patient’s lack of quitting success. Stigma involves negative attitudes, stereotypes, and judgments that society places on an individual or group based on distinguishing characteristics deemed tainted or undesirable.21,22 Stigma is an external process based in the social environment; however, stigma is often felt and internalized.23 Health-related stigma is a specific type of stigma that occurs when an individual is blamed, rejected, or devalued on the basis of a health problem or condition.24 According to attribution theory, people are more likely to stigmatize others for health problems they believe are attributable to the individual’s own behavior.25,26 In line with this theory, smokers experience stigma as a result of their tobacco use, which is often seen as a behavioral choice,27,28 and cancer patients whose disease is thought to be behaviorally mediated by smoking (eg, lung cancer patients) experience higher levels of stigma than individuals with cancers that are less commonly seen as smoking related (eg, breast cancer patients).26,29
Stigma contributes to poor health outcomes among cancer patients.30 For example, primary care physicians are shown to be less likely to refer patients with lung cancer, as compared to breast cancer, for further treatment.31,32 Moreover, cancer patients with a smoking history are more likely than never-smokers to internalize cancer-related stigma and blame themselves for their illness.33–35 Internalized stigma could delay cancer diagnosis and impede cancer treatment adherence by preventing smokers from seeking cancer screening or disclosing symptoms to providers.26,36,37 Internalized stigma in cancer patients who are current or former smokers is associated with greater shame and self-blame for their illness, which in turn contributes to poorer mental health outcomes including decreased quality of life, and greater anxiety and depression symptoms.38–41 This internalized stigma might also render cancer patients less likely to disclose their smoking status and receive smoking cessation treatment.36,42
Taken together, extant research suggests that the stigma experienced by cancer patients with a smoking history may have psychological and behavioral implications that contribute to poorer health outcomes, including continued smoking. A better understanding of the stigma experienced by individuals diagnosed with cancer is needed in order to ultimately identify ways to reduce this stigma and, thereby, associated negative health effects. In January 2012, an online news article43 reported on our previous study of continued smoking rates following lung and colorectal cancer diagnosis.8 This news article generated 411 written comments on the articles’ public discussion board, providing a unique opportunity to explore publicly shared perspectives on cancer patients who continue smoking following a cancer diagnosis.
The analysis of social media data for health-related research is indeed a promising new approach that lends itself to systematic scientific inquiry.44 Several recent studies have conducted content or linguistic analyses of data from various social media outlets (eg, Twitter and YouTube) to assess perspectives on public health issues.45–50 Several studies have also specifically analyzed comments from public discussion boards of online news articles to explore public health issues.51–56 We are not aware of any research to date, which has used this approach to explore stigma related to smoking and cancer.
The purpose of the current study was to use publicly available social media data from the news coverage of our recent article8 to qualitatively explore publicly shared perspectives on cancer patients who smoke, and develop a theoretical framework that can explain factors underlying the development of stigmatic as compared to sympathetic attitudes toward these patients.
Methods
Similar to previous studies, we conducted a document analysis of comments from the public discussion board of the online news article using principles of grounded theory.57–59 Grounded theory is an inductive research methodology that involves identifying key concepts or categories in a dataset and subsequently developing a theoretical model to explain the relationship between the categories.60,61 This approach is therefore an ideal methodology for addressing our study aim of utilizing naturalistic social media data to gain insight into factors that influence attitudes toward cancer patients who smoke. Through the use of grounded theory methods, we created a framework to explain stigmatic attitudes by applying existing categories of an attitude formation model.
All original comments (posted January 1, 2012–March 4, 2012) were copied from the article’s discussion board and pasted into a text document for review (N = 411). Usernames were removed to reduce bias. Several steps were taken to assure that the data selected for the analyses contained content relevant to the study aims. Comments that did not specifically reference cancer were removed, and comments that included unrelated opinions about smoking, or unrelated opinions in response to another comment, were deleted, resulting in a total of 139 comments. Comments that were made in response to an earlier comment that did include relevant content were included in the analyses; thus, comments did not need to be freestanding. A total of 114 unique individuals contributed to the 139 comments included in the analyses. The mean number of comments made by an individual was 1.22 (SD = .62). Ninety-seven individuals left one comment; 12 people left 2 comments; 3 people left 3 comments; 1 person left 4 comments; and 1 person left 5 comments.
To analyze the data, the study team (KH, JS, and BT) first read through each original comment to familiarize themselves with the general content and tone of the commentary. Two members of the study team then individually reviewed each comment in detail in order to identify key themes present across the comments. When possible, responders’ smoking status was identified based on the content of their comment. We only reported background details for information that was directly and explicitly stated by the writer. A list of key thematic categories, as well as subcategories for each theme, was generated. Once this coding framework was developed, each coder independently rereviewed each original comment to determine which of the identified themes were present; it was possible for a given comment to reflect themes from more than one category. The coders then compared their results and, when a discrepancy was identified, engaged in a collaborative dialogue with a senior investigator (EP) to reach a final determination.
Next, the study team assessed the recurrent themes and subcategories to identify relationships between categories. Patterns and directionality in these relationships were explored in order to develop a conceptual model of factors that influence the development of stigmatic as compared to sympathetic attitudes toward cancer patients who are current or former smokers at the time of diagnosis. Model linkages were formed by considering how each of the identified observations theoretically related to one another based on established cognitive–behavioral theories and social attribution theories.62,63 The study team compared the model to the original comments, and the model underwent a lengthy and iterative development process to ensure that the categories in the final model accurately captured the themes, and relationship between themes, as described in the original commentary. Individual comments did not need to reflect all themes included in the model; rather, themes were assessed across comments to generate a model based on patterns in the overall data. The institutional review board deemed these analyses exempt from institutional review board approval.
Results
Identified Themes and Subcategories
Personal Experiences
Many people explicitly shared their personal experiences with smoking and/or cancer. Notably, 61 comments specifically referenced lung cancer, and only 1 comment specifically referenced colorectal cancer. Specific subcategories of personal experiences were coded accordingly and included (1) personal smoking status and cancer history, (2) having a family member with a smoking or cancer history, and (3) having a friend or acquaintance with a smoking or cancer history. One person wrote, “I was diagnosed with lung cancer about a year ago. Before the diagnosis I continued to smoke and had the opinion that ‘if I have it, it’s too late to quit now’. Well I did quit just about 2 weeks before the confirming diagnosis.” Another wrote, “My dad kept smoking after treatment for throat cancer ... even after radiation and chemo he still continued.”
Statistical Literacy
Another theme identified throughout the comments was statistical literacy. Statistical literacy refers to an individual’s ability to access, understand, and utilize numerical health-related information.64,65 Many commenters expressed confusion or misunderstanding of the article’s statistics. One commenter stated, “This doesn’t make sense. If 15 percent of lung cancer sufferers never smoked a day in their lives then how [are] 90% of cases caused by active smoking?” Others interpreted the statistics in a way that supported their beliefs about smoking and cancer; one person wrote, “With these numbers, it looks that smoking is not a major cause for lung cancer.” Some commenters used their personal experiences to guide their understanding of the statistics, with one commenter stating, “I didn’t really understand the numbers … I have met more non-smokers who got cancer than not.”
Cancer Causes and Outcomes
Many comments expressed people’s beliefs about cancer, including causes of cancer and inevitable outcomes of cancer diagnoses. Some people mentioned the multiple causes of cancer beyond smoking. One person whose friend was a former smoker who died of lung cancer wrote, “Everyone ran around clucking ‘another smoking related death!’ It didn’t matter that he had been living in a house wrapped in asbestos for the last 50 years!” Other commenters argued a more direct link between smoking and cancer; one person wrote, “Lung cancer: For more information, continue smoking.” In terms of cancer outcomes, a fatalistic subcategory was identified wherein the commenter expressed concerns that cancer leads to death or a shortened life. One person stated, “Treatment for lung cancer pretty much just delays the inevitable. With few exceptions, people with the diagnosis of lung cancer will not have long to live.” Another person wrote, “You smoke, you get cancer, your fault, no treatment, you die. Simple as that.”
Beliefs About Quitting
Another identified theme involved beliefs about quitting smoking. Here, we identified two specific subcategories: (1) beliefs about the benefits of quitting post-diagnosis and (2) and beliefs that quitting is difficult because smoking involves an addiction to nicotine. For beliefs about the benefits of quitting, some people expressed that quitting post-diagnosis is futile, as one individual stated, “So what? I mean if you’ve already got cancer, why quit?” Others highlighted benefits of quitting post-diagnosis; one former smoker wrote, “I was diagnosed with throat cancer ... that is the day I quit smoking ... years later I am alive and have enjoyed watching my daughters grow up.” For beliefs about smoking as an addiction, a former smoker and cancer patient stated, “Because smoking is both a physical AND psychological addiction, it’s something you really have to focus on ... cancer treatments just do not allow you time or energy to work though the ‘quit smoking’ process.” In contrast, others discounted the role of addiction and highlighted personal failures (eg, unintelligent and weak) as reasons for smoking, with one person stating, “It’s a disgusting, deadly habit that harms everyone; when will people wise up?”
Beliefs About Responsibility
People also discussed their beliefs about who is responsible for an individuals’ continued smoking. Specifically, we identified two subcategories of smoking responsibility, reflecting (1) intrinsic and (2) extrinsic processes. Intrinsic processes focused on the smoker’s own behavior and included comments that were either sympathetic (eg, by emphasizing smokers’ personal choice and empowering them to quit) or stigmatizing to smokers (eg, by blaming smokers and questioning their intelligence for not quitting). Twenty-one comments expressed sympathy and 26 comments expressed stigma. One former smoker stated, “I had to quit ... I used Chantix ... and, thank God, I was able to continue not smoking. It was one of the toughest things I ever had to do. I don’t put anybody down for not being able to quit ... I just pray for them.” In contrast, another former smoker said, “I can’t even believe I had smoked as it is one of the most repulsive things I can think of now ... Risk of a horrible death from many forms of cancer ... You can’t afford to retire but you can afford 1 or more packs of cigs a day? Burden on others. Why do this to your family?”
Extrinsic reasons for smoking involved placing blame on big tobacco companies and the government for not banning tobacco. One person wrote, “I will never give the tobacco farmers or companies any slack ... they all know they are selling a product that is addictive and has the potential to kill. Only an immoral, unethical person would do such a thing,” and another stated, “That the FDA has not classified it as a narcotic or a controlled substance is bewildering ... smoking kills—just ban it.”
Conceptual Framework
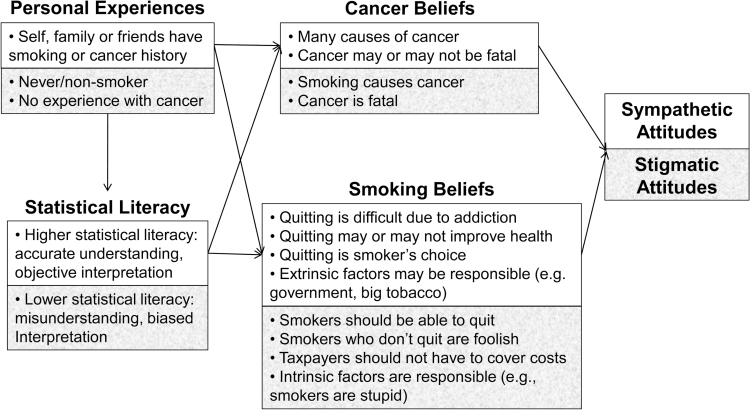
Based on the identified themes and subcategories, a conceptual framework emerged in which personal experience and statistical literacy influenced beliefs about smoking and cancer, which in turn influenced attitudes toward cancer patients who smoke. Figure 1 depicts this conceptual framework and details the specific factors that influenced sympathetic and stigmatizing attitudes.
Figure 1.
Conceptual model of themes influencing stigmatic and sympathetic attitudes. White background reflects sympathetic pathway and gray background reflects stigmatic pathway.
Individuals with personal experiences either as a current or as a former smoker, or with friends or with family who smoke, tended to express beliefs that smoking is a difficult addiction to break, external factors have some responsibility for individuals’ smoking, and there are other causes of cancer beyond smoking; thus, these individuals expressed more sympathetic and empowering attitudes toward individuals who continue to smoke following a cancer diagnosis. One former smoker stated, “Quitting smoking is a tough thing to do ... I tried it several times. ... Wound up with cancer ... I have now been cigarette free, and tobacco free ... and plan to stay that way. It can be done, and I hope anyone that tries succeeds.” Someone with a relative who was a former smoker and died of cancer stated, “[He] continued to smoke after his diagnosis. It was his choice ... I’m amazed at how judgmental some people are ....”
There were some exceptions to this pattern. First, when personal experiences involved the loss of a loved one to smoking-related cancer, individuals tended to express fatalistic beliefs about cancer outcomes. For example, one person whose father underwent multiple surgeries for smoking-related medical problems wrote, “I swear there must be a part of the brain that becomes affected that allows [smokers] to deny that smoking is what’s causing their health problems.” Second, the attitudes of former smokers were occasionally mixed: while most expressed sympathy for the difficulty in quitting and empowered smokers to quit, others demonstrated beliefs that because they were able to quit, others should be able to quit as well, resulting in judgmental and stigmatic perspectives. One individual wrote, “Anyone who smokes now is just stupid and should be made to watch people with lung cancer and the horrible death it is. I was one of those stupid people ... and can’t even believed I had smoked as it is one of the most repulsive things I can think of now.”
Individuals who did not report any personal experiences with smoking, and those who did not understand the statistics presented in the original argument, tended to believe that smoking necessarily and inevitably causes cancer and that there was little benefit to quitting post-diagnosis. These individuals also tended to focus primarily on intrinsic reasons for smoking, though they largely discounted the role of nicotine addiction and, instead, cited smokers’ “foolishness” or lack of effort as the reason for their continued smoking. One person stated, “Typical smoker thinking: ‘I don’t think smoking is causing any problems for me’. “You have cancer. ‘It’s too late to quit now, so I’ll keep doing it’. And we thought the human race was intelligent.” Conversely, individuals who believed that cancer had multiple causes tended to express greater sympathy: “[These comments] are exactly why smokers have been degraded [and] discriminated against ... People have been dying of cancers from other exposures forever, but every time a cancer report comes out it is immediately blamed on smoking.”
Discussion
Cancer patients who are current or former smokers experience significant social stigma that can negatively impact their physical and mental health. We used an innovative approach of analyzing publicly available social media data to develop a conceptual model explaining individuals’ stigmatic or sympathetic reactions to cancer patients who smoke. Previous studies have used discussion board data from an online news article to explore public health issues,51–54 though this is the first study we are aware of to apply this approach to stigma among cancer patients who smoke. Overall, we found that personal experiences with cancer and smoking, and statistical literacy, influenced beliefs about smoking and cancer, which in turn influenced attitudes toward cancer patients who smoke.
Current smokers usually did not blame cancer patients for smoking. Current smokers often considered other processes that might be responsible for continued tobacco use and expressed support for “smoker’s rights” to choose whether or not they want to quit. For the most part, former smokers acknowledged the difficulty in quitting and empathized with cancer patients who continue to smoke, drawing from their personal experiences with the difficulty of quitting. However, sometimes former smokers also stigmatized or blamed patients for not being able to quit as they had done. Never-smokers, particularly those who did not report having any friends or family who were smokers or cancer patients, usually expressed stigmatic attitudes, demonstrated a lack of understanding of addiction, focused on the direct link between smoking and cancer (ie, to the exclusion of other potential causes of cancer), and used numbers and statistics erroneously to support their views.
The finding that personal experiences influence beliefs and attitudes is consistent with social psychological theories. These findings reflect the fundamental attribution error, which posits that individuals tend to attribute their own behavior to external causes but attribute others’ behavior to internal factors.66 Thus, current or former smokers may be more sympathetic because they already evaluated the external causes for their own smoking behavior, leading them to place less judgment or blame on others for smoking. Conversely, individuals without personal experience may tend toward stigmatization because they are more likely to focus on internal causes of smoking. Indeed, Weiss et al.67 found that lung cancer patient advocates were more likely to know someone who was affected by cancer and more likely to believe that factors other than smoking (ie, genetics) were involved in the development of lung cancer. Thus, the model identified in the current study is consistent with well-established theories of attitude formation, but it applies this theory to a new area of public health.
Stigma may make individuals more vulnerable to experiencing anxiety and depression symptoms which, in turn, may have an important effect on continued smoking. Indeed, greater negative affect is associated with nicotine dependence and potentially greater smoking among cancer patients.68 Smokers consistently report that smoking helps them cope with emotional distress, that negative affect reduction is a primary smoking motive, and that they use cigarettes to manage negative emotions. As a result, increases in negative affect are one of the most powerful contributors to smoking relapse, as smokers turn back to cigarettes to manage negative emotions that come up during a quit attempt.69–73 For cancer patients who continue to smoke post-diagnosis, the emotional distress of their disease combined with the psychological consequences of being stigmatized for their health problem might interact to create unique challenges for quitting smoking.
There are several implications of the current findings. First, these findings help raise awareness of stigma toward cancer patients who smoke and provide insight into the processes that may influence stigmatic attitudes toward these patients. Results suggest that modifying cancer- and smoking-related beliefs may be helpful for cultivating sympathy and reducing stigma toward cancer patients with a smoking history. Utilizing population-based strategies, such as developing communication efforts for educating the public regarding the nature of addiction and health benefits of quitting smoking after a cancer diagnosis, may be one useful approach. Indeed, the potential for current public health interventions to create long-term negative health consequences by promoting stigma against smokers have recently been emphasized.74,75 Although stigma could be a potential motivator for smokers to quit smoking or switch to other forms of tobacco76 (eg, smokeless tobacco and e-cigarettes), our focus is on decreasing the deleterious effects of stigma. Media efforts that portray smokers’ cessation success stories rather than aim to scare smokers into quitting might be beneficial for promoting cessation while also minimizing stigma.
At the individual level, exercises that ask individuals to imagine how they would treat or feel about a friend or family member with a history of smoking and cancer might be an ethical way of leveraging the role of personal experiences. Training in mindfulness meditation has also been shown to enhance empathy for others and reduce stigma toward devalued groups77,78 as well as enhance self-compassion among cancer patients.79 Implementing mindfulness-based interventions in hospital settings for providers, patients, and their families might help to reduce sources of external stigma and patients’ own internalized stigma.80,81 Given that education strategies are most effective for people who already feel connected to the patient,82 loving kindness meditations that ask individuals to imagine feelings of warmth, compassion, and connection to others could help facilitate a sense of connectedness to cancer patients who smoke83 to maximize the benefits of education strategies.
The current findings have implications for future research. First, the proposed conceptual model should be tested empirically to further inform public health and clinical interventions. Further model validation could be done using qualitative and quantitative techniques. Specifically, research should test the salience of particular model constructs and assess sociodemographic and psychological moderators of different pathways to better target future interventions that may be developed based on this model. Qualitatively, another study could use purposive sampling techniques to target participant selection for interview on development of these attitudes. Lastly, empirical tests of this model should compare results among individuals who do and do not participate in online discussion boards to assess the generalizability of the model.
These findings should be considered in light of the strengths and limitations of analyzing data from public news discussion boards. There are several benefits to this approach. These discussion boards are accessible to many people, have the potential to reach broad geographical areas, and allow the public to naturally and spontaneously voice their opinions and engage in discourse; as such, they provide a substantial source of qualitative data that is current and readily available at no cost to researchers.55,84 Additionally, there is a decreased risk of social desirability bias due to anonymity, which is particularly relevant to stigma research.53 Anonymity allows individuals to express opinions about sensitive health issues without anxiety, fear of rejection, and pressure to confirm, ultimately leading to more honest opinions that might not be expressed in face-to-face interactions.53,85,86 However, it can also lead to the expression of hostile or aggressive comments,87 which is important because online discussions and the emotional tone of online comments can themselves affect readers’ attitudes and behaviors.51,54,88–90
It is therefore important to understand to what extent stigmatic attitudes are expressed and propagated online. As such, a novel implication of the current study is that online discussions about cancer and smoking may be contributing to stigma but are also contributing perspectives that are sympathetic, as evidenced by only slightly more stigmatic comments as compared to sympathetic comments.
These benefits are not without limitations. A key limitation is that this approach precludes the opportunity for systematic sociodemographic data collection and prevented us from being able to collect additional data during the theoretical model development stage. Although recent research suggests that social media communication produces valid and reliable information,48,91,92 precise information regarding the demographic and clinical characteristics of respondents is lacking, and it is not possible to assess the specific factors that might influence one’s attitudes toward these patients (eg, factors that differentiate sympathetic former smokers from stigmatic former smokers).51,53,55 Additional qualitative exploration using purposive sampling of selected demographic characteristics would be beneficial to further assess the model. Additionally, respondents were a self-selected group who likely have strong opinions and thus may differ from other individuals, potentially limiting the generalizability of the current findings.53,93 At this stage, our aim was to leverage the advantages of anonymous social media data to develop an unbiased conceptual model; the overall utility of this model and its generalizability to different demographic groups and non-Internet users is a necessary next step for future research.
Ethical considerations of social media data analysis also require further study. To our knowledge, there is currently no consensus regarding complex issues such as anonymity, privacy, and informed consent.44,53 Although the news site used for the current data did not indicate that comments may be used for research, it has been suggested that individuals who post on public forums do not have an expectation that their comments are private, and we preserved anonymity by not reporting commenters’ usernames or personally identifiable information.44
Another limitation to this study is that we were unable to directly assess differences in stigma toward lung versus colorectal cancer patients. However, the fact that many of the comments were focused on lung cancer might itself be a reflection of the greater levels of smoking-related stigma that lung cancer patients experience, compared to other cancer patients.26 That is, even though the original news article reported on both lung and colorectal cancer patients, respondents tended to discuss smoking issues in terms of lung cancer patients specifically. In general, research on stigma in colorectal patients is lacking in comparison with the research on stigma in lung cancer patients, and it would be worthwhile to assess whether the processes that influence the development of stigma vary based on cancer diagnosis, particularly when the cancers are more or less seen as smoking related. Lastly, while the current study provides insight into publicly shared perspective, future studies should assess the impact of stigma from the patient’s perspective. Indeed, a recent qualitative study found that lung cancer patients experience a unique emotional burden from their illness due to stigma, societal attitudes, and shame and self-blame.94 Further research should assess the ways in which stigma may impact emotional distress and smoking cessation efforts during or after cancer treatment across different cancer groups.
Overall, the current findings add to the literature on smoking, cancer, and stigma by elucidating factors that may be relevant to the development of stigma against smokers with cancer. Future research should assess the effects of stigma-related distress on smoking behavior and cessation efforts among cancer patients post-diagnosis. Smoking cessation programs for cancer patients and survivors should highlight the nature of addiction and difficulty of quitting to help reduce stigma and combat the negative impact stigma may have on smokers’ quitting and health outcomes. These programs should also be sure to offer first-line behavioral and pharmacological smoking cessation treatment to cancer patients in order to promote quitting.
Funding
This study was supported by funds from the National Center for Complementary and Integrative Health (NCCIH 2T32AT000051-6 to CML) and National Cancer Institute (1K24CA197382 to ERP)
Declaration of Interests
None declared.
References
- 1. Services USDoHaH. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2014. [Google Scholar]
- 2. Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48(3):326–333. doi:10.1016/j.amepre.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 4. Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat. 2010;10(249):1–207. [PubMed] [Google Scholar]
- 5. Services USDoHaH. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Disease Prevention and Health Promotion, Office of Smoking; 2005. [Google Scholar]
- 6. Society AC. Cancer Facts and Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 7. Prevention CfDCa. Quitting Smoking Among Adults-United States. MMWR Morb Mortal Wkly Rep. 2011; 60(44):1513–1519. [PubMed] [Google Scholar]
- 8. Park ER, Japuntich SJ, Rigotti NA, et al. A snapshot of smokers after lung and colorectal cancer diagnosis. Cancer. 2012;118(12):3153–3164. doi:10.1002/cncr.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dresler CM. Is it more important to quit smoking than which chemotherapy is used? Lung Cancer. 2003;39(2):119–124. [DOI] [PubMed] [Google Scholar]
- 10. Garces YI, Schroeder DR, Nirelli LM, et al. Second primary tumors following tobacco dependence treatments among head and neck cancer patients. Am J Clin Oncol. 2007;30(5):531–539. [DOI] [PubMed] [Google Scholar]
- 11. Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi:10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79(2):414–419. doi:10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 13. McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control. 2003;10(4):325–333. [DOI] [PubMed] [Google Scholar]
- 14. Bjurlin MA, Goble SM, Hollowell CM. Smoking cessation assistance for patients with bladder cancer: a national survey of American urologists. J Urol. 2010;184(5):1901–1906. [DOI] [PubMed] [Google Scholar]
- 15. Cooley ME, Emmons KM, Haddad R, et al. Patient-reported receipt of and interest in smoking-cessation interventions after a diagnosis of cancer. Cancer. 2011;117(13):2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simmons VN, Litvin EB, Unrod M, Brandon TH. Oncology healthcare providers’ implementation of the 5A’s model of brief intervention for smoking cessation: patients’ perceptions. Patient Educ Couns. 2012;86(3):414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17(3):455–462. doi:10.1634/theoncologist.2011-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobacco cessation and quality cancer care. J Oncol Pract. 2009;5(1):2–5. doi:10.1200/JOP.0913501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker MS, Larsen RJ, Zona DM, Govindan R, Fisher EB. Smoking urges and relapse among lung cancer patients: findings from a preliminary retrospective study. Prev Med. 2004;39(3):449–457. [DOI] [PubMed] [Google Scholar]
- 20. Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2370–2377. [DOI] [PubMed] [Google Scholar]
- 21. Goffman E. Stigma: Notes on the Management of Spoiled Identity. Englewood Cliffs, NJ: Prentice-Hall; 1963. [Google Scholar]
- 22. Link BG, Phelan JC. Stigma and its public health implications. Lancet. 2006;367(9509):528–529. [DOI] [PubMed] [Google Scholar]
- 23. Major B, O’Brien LT. The social psychology of stigma. Annu Rev Psychol. 2005;56:393–421. [DOI] [PubMed] [Google Scholar]
- 24. Weiss MG, Ramakrishna J. Stigma interventions and research for international health. Lancet. 2006;367(9509):536–538. [DOI] [PubMed] [Google Scholar]
- 25. Kelley HH, Michela JL. Attribution theory and research. Annu Rev Psychol. 1980;31:457–501. [DOI] [PubMed] [Google Scholar]
- 26. Lebel S, Devins GM. Stigma in cancer patients whose behavior may have contributed to their disease. Future Oncol. 2008;4(5):717–733. doi:10.2217/14796694.4.5.717. [DOI] [PubMed] [Google Scholar]
- 27. Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16(3):264–269. doi:10.1016/j.ejon.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verger P, Arnaud S, Ferrer S, et al. Inequities in reporting asbestos-related lung cancer: influence of smoking stigma and physician’s specialty, workload and role perception. Occup Environ Med. 2008;65(6):392–397. [DOI] [PubMed] [Google Scholar]
- 29. Marlow LA, Waller J, Wardle J. Does lung cancer attract greater stigma than other cancer types? Lung Cancer. 2015;88(1):104–107. doi:10.1016/j.lungcan.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 30. Chambers SK, Dunn J, Occhipinti S, et al. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 2012;12:184. doi:10.1186/1471-2407-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rasco DW, Yan J, Xie Y, Dowell JE, Gerber DE. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5(10):1529–1535. doi:10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wassenaar TR, Eickhoff JC, Jarzemsky DR, Smith SS, Larson ML, Schiller JH. Differences in primary care clinicians’ approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2(8):722–728. [DOI] [PubMed] [Google Scholar]
- 33. Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH, Lee SJ. Stigma among patients with lung cancer: a patient-reported measurement model. Psychooncology. 2014;23(1):81–92. doi:10.1002/pon.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Else-Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009;24(8):949–964. doi:10.1080/08870440802074664. [DOI] [PubMed] [Google Scholar]
- 35. LoConte NK, Else-Quest NM, Eickhoff J, Hyde J, Schiller JH. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clin Lung Cancer. 2008;9(3):171–178. doi:10.3816/CLC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 36. Carter-Harris L, Hermann CP, Schreiber J, Weaver MT, Rawl SM. Lung cancer stigma predicts timing of medical help-seeking behavior. Oncol Nurs Forum. 2014;41(3):E203–E210. doi:10.1188/14.ONF.E203-E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stuber J, Galea S. Who conceals their smoking status from their health care provider? Nicotine Tob Res. 2009;11(3):303–307. doi:10.1093/ntr/ntn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown C, Cataldo J. Explorations of lung cancer stigma for female long-term survivors. Nurs Inq. 2013;20(4):352–362. doi:10.1111/nin.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cataldo JK, Brodsky JL. Lung cancer stigma, anxiety, depression and symptom severity. Oncology. 2013;85(1):33–40. doi:10.1159/000350834. [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez BD, Jacobsen PB. Depression in lung cancer patients: the role of perceived stigma. Psychooncology. 2012;21(3):239–246. doi:10.1002/pon.1882. [DOI] [PubMed] [Google Scholar]
- 41. Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health. 2013;103(5):813–821. doi:10.2105/AJPH.2012.301069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carter-Harris L. Lung cancer stigma as a barrier to medical help-seeking behavior: Practice implications. J Am Assoc Nurse Pract. 2015;27(5): 240–245. doi:10.1002/2327-6924.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Society of Clinical Oncology. Many keep smoking after cancer diagnosis. 2012. http://vitals.nbcnews.com/_news/2012/01/23/10201507-many-keep-smoking-after-cancer-diagnosis?lite Accessed March 4, 2016.
- 44. Kraut R, Olson J, Banaji M, Bruckman A, Cohen J, Couper M. Psychological research online: report of Board of Scientific Affairs’ Advisory Group on the Conduct of Research on the Internet. Am Psychol. 2004;59(2):105–117. [DOI] [PubMed] [Google Scholar]
- 45. Corley CD, Cook DJ, Mikler AR, Singh KP. Text and structural data mining of influenza mentions in Web and social media. Int J Environ Res Public Health. 2010;7(2):596–615. doi:10.3390/ijerph7020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Choundhury M, Gamon M, Counts S, Horvitz E. Predicting depression via social media. In: Proceedings of 7th International AAAI Conference on Weblogs and Social Media; July 8–10, 2013; Boston, MA. [Google Scholar]
- 47. Chou WY, Hunt Y, Folkers A, Augustson E. Cancer survivorship in the age of YouTube and social media: a narrative analysis. J Med Internet Res. 2011;13(1):e7. doi:10.2196/jmir.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheong F, Cheong C. Social Media Data Mining: A Social Network Analysis of Tweets During the 2010–2011 Australian Floods. In: 15th Pacific Asia Conference on Information Systems: Quality Research in Pacific; July 9, 2011; Minxiong Township, Chiayi County, Taiwan. [Google Scholar]
- 49. Ceron A, Curini L, Iacus SM. Using sentiment analysis to monitor electoral campaigns: method matters: evidence from the United States and Italy. Soc Sci Comput Rev. 2014;33(1):3–20. [Google Scholar]
- 50. Oh O, Agrawal M, Rao HR. Anxiety and rumor: exploratory analysis of Twitter posts during the Mumbai terrorist attack. In: Dalziel G, ed. The Political and Social Impact of Rumor. Singapore: S. Rajaratnam School or International Studies, Nanyang Technological Univeristy; 2010:143–155. [Google Scholar]
- 51. Brown-Johnson C, Sanders-Jackson A, Prochaska JJ. Public commenting on inpatient psychiatry smoking ban media coverage. J Dual Diag.2014;10(4):204–211. doi:10.1080/15504263.2014.961883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oliver J, Thomson G, Wilson N. Public attitudes to new smokefree outdoor places policies: an analysis of 217 New Zealand online comments. N Z Med J. 2014;127(1392):109–111. [PubMed] [Google Scholar]
- 53. De Brún A, McCarthy M, McKenzie K, McGloin A. Weight stigma and narrative resistance evident in online discussions of obesity. Appetite. 2014;72:73–81. doi:10.1016/j.appet.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 54. Glenn NM, Champion CC, Spence JC. Qualitative content analysis of online news media coverage of weight loss surgery and related reader comments. Clin Obes. 2012;2(5-6):125–131. [DOI] [PubMed] [Google Scholar]
- 55. Feinberg Y, Pereira JA, Quach S, et al. Understanding public perception of the HPV vaccination based on online comments to Canadian news articles. PLoS One. 2015;10(6):1–13. doi:10.1371/journal.pone.0129587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pereira JA, Quach S, Dao HH, et al. Contagious comments: What was the online buzz about the 2011 Quebec measles outbreak. PLoS One. 2013; 8(5):1–9. doi:10.1371/journal.pone.0064072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bowen GA. Document analysis as a qualitative research method. Qual Res J 2009;9(2):27–40. [Google Scholar]
- 58. D Milioni KV, Papa V. Their two cents worth: exploring user agency in readers comments in online news media. Observatorio. 2012;6(3):21–47. [Google Scholar]
- 59. Anselm Strauss JC. Grounded theory methodology: an overview. In: Norman K, Denzin YSL, eds. Handbook of Qualitative Research. Thousand Oaks, CA: Sage; 1994:273–285. [Google Scholar]
- 60. Anselm Strauss JC. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 2nd ed. London, UK: Sage Publications; 1998. [Google Scholar]
- 61. Glaser BJ, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967. [Google Scholar]
- 62. Beck JS. Cognitive Therapy. Malden, MA: John Wiley & Sons, Inc; 1979. [Google Scholar]
- 63. Weiner B. An attributional theory of achievement motivation and emotion. Psychol Rev. 1985;92(4):548–573. [PubMed] [Google Scholar]
- 64. Centers for Disease Control and Prevention. Understanding Literacy and Numeracy 2015. www.cdc.gov/healthliteracy/learn/understandingliteracy.html Accessed March 4, 2016.
- 65. Gigerenzer G, Gaissmaier W, Kurz-Milcke E, Schwartz LM, Woloshin Helping doctors and patients make sense of health statistics. Psychol Sci. 2007;8(2):53–96. [DOI] [PubMed] [Google Scholar]
- 66. Jones EE, Nisbett REN. The actor and the observer: divergent peceptions of the causes of behavior. In: Jones EE, Kanouse D, Kelly HH, Nisbett RE, Valins S, Weiner B, eds. Attribution: Perceiveing the Causes of Behavior. Morristown, NJ: General Learning Press; 1972:79–94. [Google Scholar]
- 67. Weiss J, Stephenson BJ, Edwards LJ, Rigney M, Copeland A. Public attitudes about lung cancer: stigma, support, and predictors of support. J Multidiscip Healthc. 2014;7:293–300. doi:10.2147/JMDH.S65153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dirkse D, Lamont L, Li Y, Simonič A, Bebb G, Giese-Davis J. Shame, guilt, and communication in lung cancer patients and their partners. Curr Oncol. 2014;21(5):e718–e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. [DOI] [PubMed] [Google Scholar]
- 70. Brandon TH, Baker TB. Smoking Outcome Expectancies Among College Students. Washington, DC: ERIC Clearinghouse; 1990. [Google Scholar]
- 71. Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol Assesment. 1995;7(4):484–494. [Google Scholar]
- 72. Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70(1):216–227. [PubMed] [Google Scholar]
- 73. Piper ME, Piasecki TM, Federman EB, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). J Consult Clin Psychol. 2004;72(2):139–154. [DOI] [PubMed] [Google Scholar]
- 74. Bayer R, Stuber J. Tobacco control, stigma, and public health: rethinking the relations. Am J Public Health. 2006;96(1):47–50. doi:10.2105/AJPH.2005.071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ritchie D, Amos A, Martin C. “But it just has that sort of feel about it, a leper”—stigma, smoke-free legislation and public health. Nicotine Tob Res. 2010;12(6):622–629. doi:10.1093/ntr/ntq058. [DOI] [PubMed] [Google Scholar]
- 76. Brown-Johnson CG, Popova L. Exploring smoking stigma, alternative tobacco product use, & quit attempts. Health Behav Policy Rev. 2016;3(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Birnie K, Garland SN, Carlson LE. Psychological benefits for cancer patients and their partners participating in mindfulness-based stress reduction (MBSR). Psychooncology. 2010;19(9):1004–1009. doi:10.1002/pon.1651. [DOI] [PubMed] [Google Scholar]
- 78. Masuda A, Hayes SC, Fletcher LB, et al. Impact of acceptance and commitment therapy versus education on stigma toward people with psychological disorders. Behav Res Ther. 2007;45(11):2764–2772. [DOI] [PubMed] [Google Scholar]
- 79. Boyle CC, Bower JE. Mind-body therapies for cancer survivors: effects of yoga and mindfulness meditation on cancer-related physical and behavioral symptoms. In: Lavretsky H, Sajatovic M, Reynolds C III, eds Complementary and Integrative Therapies for Mental Health and Aging. Oxford, UK: Oxford University Press; 2016. doi:10.1093/med/9780199380862.001.0001. [Google Scholar]
- 80. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61–66. doi:10.1016/j.ctcp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 81. Lillis J, Hayes SC, Bunting K, Masuda A. Teaching acceptance and mindfulness to improve the lives of the obese: a preliminary test of a theoretical model. Ann Behav Med. 2009;37(1):58–69. doi:10.1007/s12160-009-9083-x. [DOI] [PubMed] [Google Scholar]
- 82. Evans-Lacko S, Gronholm PC, Hankir A, Pingani L, Corrigan P. Practical strategies to fight stigma in mental health. In: Fiorillo A, Volpe U, Bhugra D, eds. Psychiatry in Practice: Education, Experience, and Expertise. 1st ed. Oxford: Oxford University Press; 2016:237–251. [Google Scholar]
- 83. Hutcherson CA, Seppala EM, Gross JJ. Loving-kindness meditation increases social connectedness. Emotion. 2008;8(5):720–724. doi:10.1037/a0013237. [DOI] [PubMed] [Google Scholar]
- 84. Rowe G, Hawkes G, Houghton J. Initial UK public reaction to avian influenza: Analysis of opinions posted on the BBC website. Health Risk Soc. 2008;10(4):361–384. doi:10.1080/13698570802166456. [Google Scholar]
- 85. Campbell M, Meier A, Carr C, et al. Health behavior changes after colon cancer: a comparision of findings from face-to-face and on-line focus groups. Fam Community Health. 2001;24(3):88–103. [DOI] [PubMed] [Google Scholar]
- 86. Wallace JD. Computer-mediated communcation research. In Zaphiris P, Ang CS, eds. Human Computer Interaction: Concepts, Methodologies, Tools, and Applications. Hersey, PA: Information Science Reference;2008:299–315 [Google Scholar]
- 87. Suler J. The online disinhibition effect. Cyberpsychol Behav. 2004;7(3):321–326. [DOI] [PubMed] [Google Scholar]
- 88. Walther JB, DeAndrea D, Kim J, Anthony JC. The influence of online comments on perceptions of antimarijuana public service announcements on YouTube. Hum Commun Res. 2010;36:469–492. [Google Scholar]
- 89. Anderson AA, Brossard D, Scheufele DA, Xenos MA, Ladwig P. The “nasty effect:” Online incivility and risk perceptions of emerging technologies. J Comput Mediat Commun. 2014; 19(3):373–387. [Google Scholar]
- 90. Walther JB, Jeong-woo J. Communication processes in participatory websites. J Comput Mediat Commun. 2012;18(1):2–15. doi:10.1111/j.1083-6101.2012.01592.x. [Google Scholar]
- 91. Mendoza M, Poblete B, Castillo C. Twitter under crisis: can we trust what we RT? In: Proceedings of First Workshop on Social Media Analytics; July 25, 2010; Washington, DC. [Google Scholar]
- 92. Castillo C, Mendoza M, Poblete B. Information credibility on Twitter. In: Proceedings of the 20th International Conference on World Wide Web; March 28–April 1, 2011; Hyderabad, India. [Google Scholar]
- 93. Holton A, Lee N, Coleman R. Commenting on health: a framing analysis of user comments in response to health articles online. J Health Commun. 2014;19(7):825–837. [DOI] [PubMed] [Google Scholar]
- 94. Lehto RH. Patient views on smoking, lung cancer, and stigma: a focus group perspective. Eur J Oncol Nurs. 2014;18(3):316–322. doi:10.1016/j.ejon.2014.02.003. [DOI] [PubMed] [Google Scholar]