Abstract
Introduction
We develop a multidomain model to predict progression of Alzheimer's disease dementia (AD).
Methods
Data from the US National Alzheimer's Coordinating Center (n = 3009) are used to examine change in symptom status and to estimate transition probabilities between health states described using cognitive function, functional ability, and behavior. A model is used to predict progression and to assess a hypothetical treatment scenario that slows mild to moderate AD progression.
Results
More than 70% of participants moved state over 12 months. The majority moved in domains other than cognitive function. Over 5 years, of those alive more than half are in severe AD health states. Assessing an intervention scenario, we see fewer years in more severe health states and a potential impact (life years saved) due to mortality improvements.
Discussion
The model developed is exploratory and has limitations but illustrates the importance of using a multidomain approach when assessing impacts of AD and interventions.
Keywords: Alzheimer's disease, Decision analytic modelling, Progression, Prediction, Health policy
1. Introduction
The world-wide prevalence of Alzheimer's disease and other dementing disorders is predicted to increase from an estimated 35.6 million in 2010 to 115.4 million in 2050 [1]. This is a clear marker for the growing importance and the future challenges related to dementia. Alzheimer's disease dementia (AD) represents an increasingly significant health care burden on individuals, families, carers, and health care systems [2,3]. The growing health care burden of AD, aligned with competing demands on available health care resources, presents health policy decision makers with challenges, many of these linked to financial and other resource use constraints associated with the development and adoption of health and social care services and also over the funding of future research. To make the case for AD requires an increasing awareness and understanding of the social and economic impact of AD and intervention strategies to support those affected by AD. In this context, decision analytic policy models are commonly used, often with a public policy remit, to investigate questions about the effectiveness and impact on cost and health-related quality of life of a wide range of health care interventions.
However, the development of methods to model the impact of AD has been relatively slow. Advances in drug therapies for AD over the 1990s led to improvements in modeling methods to support claims for the adoption of these new treatments. Earlier reviews [4,5] report that modeling methods in AD have often used a simplified representation of the underlying disease and symptom structure for AD, commonly considering only cognitive function, and that these models may have limitations in identifying the impact of interventions for AD [4,5]. In recent years, we have seen a continued growth in the evidence base on the methods available to model the progression of AD; yet, there remains a reliance on the use of cognitive function in the assessment of the impacts of AD and the likely effectiveness of intervention strategies (see Supplementary Table A).
Although cognitive function is a defining and central factor in AD, the original description of AD included behavioral and psychotic symptoms along with memory loss [6]. The diagnosis of AD includes requirements for both cognitive impairment and substantial impairment in daily functioning or behavioral symptoms (NINCDS-ADRA) [7]. Changes in one or more of these domains provide the basis for assessing severity and progression of AD [8]. However, research on the modeling of AD has emphasized cognitive decline over loss of functional ability and behavioral symptoms, such as psychosis, agitation and depression. This common conceptualization, with cognition often as the only predictive variable, may limit our ability to estimate the impact of AD and the potential benefits of intervention strategies.
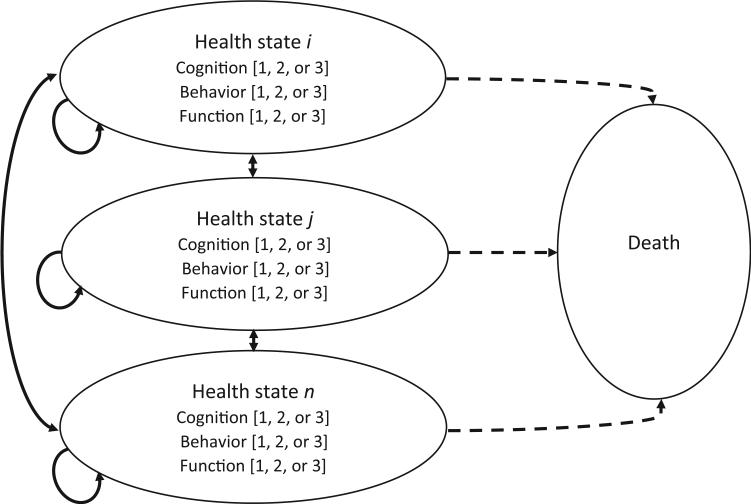
Results from recent multivariate research in AD [9] recommend that the most appropriate approach to model AD and its progression involves incorporating the symptom domains of cognition, behavior, and function. Responding to such recommendations, we take a simple conceptual multi-domain model of AD progression (See Fig. 1 [4]), using these three symptom domains, and operationalize this model using available data. The aim was to demonstrate the utility of a multidomain approach to modeling progression of AD and to provide a foundation from which to develop a model to support the health policy context.
Fig. 1.
Schematic for Multi Domain AD Progression Model. The figure presents the conceptual model and schematic for the multi domain AD progression model, showing AD health sates (states i, j, ...n) described using cognitive function, behavior, and functional ability, with each domain described using three levels (categories of severity). Death is a health state (absorbing state) in the model.
2. Methods
2.1. Participants
We use the Uniform Data Set (UDS) from the US National Alzheimer's Coordinating Center (NACC) [10,11]. The UDS contains data from the Alzheimer disease centers (ADCs) across the United States. All ADCs enroll and follow participants annually and provide pooled data for research through the NACC. Diagnoses are assigned using clinical criteria. The NACC UDS includes data on cognitive function, functional impairment, and behavior; using the Mini-Mental State Examination (MMSE) [12], Functional Assessment Questionnaire (FAQ) [13], and the Neuropsychiatric Inventory (NPI) [14]. We limited data analyses to those participants who were aged ≥50 years, have an MMSE score of ≤26, and have continuous data collected over time on at least two consecutive assessments, including a diagnosis of probable AD and MMSE data. We included participants with a diagnosis of probable AD on entry to the data set, and also those that were known to ADCs before having a diagnosis of probable AD. We extracted data from eligible participants with complete data on MMSE, FAQ, and NPI-Q in March 2014.
2.2. Descriptive system for Alzheimer's dementia
A multidomain descriptive system is used, capturing the three primary symptom domains of AD; cognitive function, behavior and mood, and functional impairment. A simple categorical approach is used to place impairments on each of the domains into one of three severity levels, covering no or mild problems, moderate problems, or severe problems (see Table 1). This approach is consistent with the typical interpretation of the rating scales used to assess symptomatic problems in AD, see below. There is a broad range of rating scales, and clinical measures available to assess health status and impairment, and here, we use the MMSE, NPI-Q, and the FAQ, as proxy measures for cognitive function, behavior and mood, and functional impairment.
Table 1.
Descriptive system for AD: Definition by level of severity for each symptom domain
| Domain | Severity level | Label | Definition |
|---|---|---|---|
| Cognitive function | Mild | [1] | 21 ≤ MMSE ≤ 26 |
| Moderate | [2] | 10 ≤ MMSE ≤20 | |
| Severe | [3] | 0 ≤ MMSE ≤ 9 | |
| Behavior & Mood | No problem/mild | [1] | NPI-Q: each item ≤ 1 |
| Moderate | [2] | NPI-Q: each item ≤ 2; with at least one item equal to 2 | |
| Severe | [3] | NPI-Q: at least one item equal to 3 | |
| Functional Ability | No problem | [1] | 0 ≤ FAQ total ≤ 8 |
| Moderate | [2] | 9 ≤ FAQ total ≤ 23 | |
| Severe | [3] | 24 ≤ FAQ total ≤ 30 |
2.2.1. MMSE
The MMSE [12] is a clinician administered measure to assess global cognitive functioning. Although there is no range of scores that can be rigidly and universally applied to indicate dementia severity, mild AD is often associated with an MMSE of 21 to 26, moderate AD with an MMSE of 10 to 20 and severe AD with an MMSE of <10. This simple categorization of AD cognitive function, as commonly used in a policy setting (e.g., UK NICE [15]) is applied here.
2.2.2. NPI-Q
The NPI-Q [14] is a brief 10-item questionnaire derived from the Neuropsychiatric Inventory (NPI) [16], a validated clinical instrument for evaluating psychopathology in dementia. It assesses 10 behavioral domains common in dementia, and each of these domains where present are scored 1–3 for severity: 1 as mild (e.g. present but not distressing/disruptive to the person with dementia), 2 moderate, and 3 as severe. Here, consistent with the approach used by Spalleta et al. [17], we categorize behavior and mood into three severity levels, with no or mild problems where all items are scored at 1 or 0, severe problems when at least one of the items is scored at 3, and moderate problems when at least one item is scored at 2, with all others at ≤2.
2.2.3. FAQ
The Functional Activities Questionnaire (FAQ) [13] is an informant-based assessment on 10 activity items for the person with dementia, with each scored on four levels (from 0–3) ranging from dependence to independence (3 = dependent, 2 = requires assistance, 1 = has difficulty but does by self, 0 = normal). The items also have a “not applicable” response category. The FAQ is scored at 0–30, derived using the mean scores for the applicable items multiplied by 10, where higher scores indicate greater functional impairment and dependence. A score of eight or below is regarded as no problems, and a score of 24 or above is indicated as being more severely affected and highly dependent [13].
2.3. Statistical analyses
Participants included in the analyses are allocated into the descriptive system defined here (Table 1), at each time point, using participant level data on each symptom domain. The mean change in domain specific score is described, and the proportion of people moving between domain specific categories is described. The frequency of moves between health states is described, and the transitions between states are calculated to derive transition probabilities between states. Transition probabilities are estimated using a fixed cohort approach, defined as (N0–Nt)/N0, where N0 represents the size of the population eligible to transit to a state at the beginning of the time interval, and Nt represents the number remaining eligible to transit at time t [18]. The size of the transition matrix precludes use of a dynamic cohort approach (where probabilities are derived from estimated rates), within the constraints of the current research.
2.4. Modeling framework
Using the transition probabilities derived here, we predict the progression of AD, applying a 1-year cycle length over a 5-year time horizon, for a hypothetical cohort with mild to moderate AD. We use a mild to moderate AD cohort as an example here as this is currently the treatment eligible population for cholinesterase inhibitors, although the modeling framework predicts separately by health state and allows prediction by specific severity categories (e.g., for mild and/or moderate AD). The starting distribution of the cohort, over eight health states, is based on observed proportions in mild to moderate AD states in the NACC data used, using mild and moderate states where no severe symptoms are present, comprising 69.5% mild and 30.5% moderate AD (see Table 2). Using estimated annual transition probabilities, we assume a transit at the mid-point of each cycle and assess two scenarios for mortality, using (1) a simplifying assumption of a standard annual mortality rate (10% pa [19–22]) across all people with AD, and (2) differential annual mortality risks by mild, moderate, and severe AD (see Table 5). This simple modeling framework is used to assess the value of the multidomain approach in a health policy context.
Table 2.
Multi domain descriptive system for AD, and participants (NACC-UDS, n = 3009) by location
| Domain severity level |
State severity label | Number at baseline | Number at 12-month follow-up | |||
|---|---|---|---|---|---|---|
| Cognition | Behavior | Function | State name | |||
| 1 | 1 | 1 | 111 | Mild* | 455 | 226 |
| 1 | 1 | 2 | 112 | Mild* | 581 | 444 |
| 1 | 1 | 3 | 113 | Moderate | 63 | 79 |
| 1 | 2 | 1 | 121 | Mild* | 101 | 44 |
| 1 | 2 | 2 | 122 | Mild* | 354 | 249 |
| 1 | 2 | 3 | 123 | Moderate | 56 | 82 |
| 1 | 3 | 1 | 131 | Moderate | 29 | 12 |
| 1 | 3 | 2 | 132 | Moderate | 97 | 84 |
| 1 | 3 | 3 | 133 | Moderate | 33 | 46 |
| 2 | 1 | 1 | 211 | Moderate* | 77 | 54 |
| 2 | 1 | 2 | 212 | Moderate* | 361 | 386 |
| 2 | 1 | 3 | 213 | Moderate | 159 | 245 |
| 2 | 2 | 1 | 221 | Moderate* | 20 | 14 |
| 2 | 2 | 2 | 222 | Moderate* | 191 | 207 |
| 2 | 2 | 3 | 223 | Moderate | 158 | 253 |
| 2 | 3 | 1 | 231 | Moderate | 5 | 5 |
| 2 | 3 | 2 | 232 | Moderate | 59 | 73 |
| 2 | 3 | 3 | 233 | Severe | 76 | 161 |
| 3 | 1 | 2 | 312 | Severe | 9 | 28 |
| 3 | 1 | 3 | 313 | Severe | 34 | 100 |
| 3 | 2 | 2 | 322 | Severe | 6 | 10 |
| 3 | 2 | 3 | 323 | Severe | 57 | 106 |
| 3 | 3 | 2 | 332 | Severe | 2 | 4 |
| 3 | 3 | 3 | 333 | Severe | 26 | 96 |
| 3 | 1 | 1 | 311 | Severe | 0 | 1 |
| 3 | 2 | 1 | 321 | Severe | 0 | 0 |
| 3 | 3 | 1 | 331 | Severe | 0 | 0 |
Columns 1–3: 1 refers to mild in cognition, no problem/mild in behavior, no problem in function; 2 refers to moderate in cognition, behavior and function; 3 refers to severe in cognition, behavior and function.
These eight health states are used to define a cohort of people with mild to moderate AD, i.e., 21% in state 111, 27% in state 112, and so on (see Table 5; showing 69.5% mild, 30.5% moderate AD). The defined cohort is used to demonstrate the application of the modeling framework in a health policy context.
Table 5.
Predicted AD progression over time (month 0–60) by group (control and hypothetical treatment cohorts) and by mortality scenario
| Proportion participants, at the end of month |
Life years (in state) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Group | Mild | Moderate | Severe | Death | Group | Mild | Moderate | Severe | Death |
| Start Distribution | 69.50 | 30.50 | 0.00 | 0.00 | ||||||
| Month 12 | C1 | 38.57 | 44.13 | 7.30 | 10.00 | C1 | 54.04 | 37.31 | 3.65 | 5.00 |
| T1 | 48.49 | 37.08 | 4.43 | 10.00 | T1 | 58.99 | 33.79 | 2.21 | 5.00 | |
| C2 | 40.50 | 37.83 | 4.22 | 17.45 | C2 | 55.00 | 34.16 | 2.11 | 8.73 | |
| T2 | 50.91 | 31.95 | 2.56 | 14.58 | T2 | 60.21 | 31.22 | 1.28 | 7.29 | |
| Month 24 | C1 | 22.46 | 42.56 | 15.98 | 19.00 | C1 | 30.52 | 43.35 | 11.64 | 14.50 |
| T1 | 27.64 | 41.24 | 12.12 | 19.00 | T1 | 38.06 | 39.16 | 8.28 | 14.50 | |
| C2 | 24.06 | 33.31 | 7.25 | 35.38 | C2 | 32.28 | 35.57 | 5.73 | 26.41 | |
| T2 | 29.85 | 33.10 | 5.71 | 31.34 | T2 | 40.38 | 32.52 | 4.14 | 22.96 | |
| Month 36 | C1 | 13.48 | 36.49 | 22.94 | 27.10 | C1 | 17.97 | 39.52 | 19.46 | 23.05 |
| T1 | 16.3 | 37.16 | 19.43 | 27.10 | T1 | 21.97 | 39.20 | 15.78 | 23.05 | |
| C2 | 14.42 | 25.98 | 8.19 | 51.41 | C2 | 19.24 | 29.65 | 7.72 | 43.39 | |
| T2 | 17.7 | 27.54 | 7.40 | 47.31 | T2 | 23.80 | 30.32 | 6.56 | 39.32 | |
| Month 48 | C1 | 8.33 | 29.75 | 27.53 | 34.39 | C1 | 10.90 | 33.12 | 25.24 | 30.75 |
| T1 | 9.9 | 30.98 | 24.71 | 34.39 | T1 | 13.12 | 34.07 | 22.07 | 30.75 | |
| C2 | 8.74 | 19.00 | 7.69 | 64.58 | C2 | 11.58 | 22.49 | 7.94 | 57.99 | |
| T2 | 10.7 | 20.86 | 7.50 | 60.97 | T2 | 14.21 | 24.20 | 7.45 | 54.14 | |
| Month 60 | C1 | 5.26 | 21.98 | 31.81 | 40.95 | C1 | 6.80 | 25.86 | 29.67 | 37.67 |
| T1 | 6.17 | 23.16 | 29.72 | 40.95 | T1 | 8.05 | 27.07 | 27.21 | 37.67 | |
| C2 | 5.33 | 12.79 | 6.90 | 74.98 | C2 | 7.03 | 15.90 | 7.29 | 69.78 | |
| T2 | 6.47 | 14.36 | 7.10 | 72.08 | T2 | 8.57 | 17.61 | 7.30 | 66.52 | |
| Total life years | ||||||||||
| C1 | 120.22 | 179.16 | 89.65 | 110.97 | ||||||
| T1 | 140.19 | 173.29 | 75.55 | 110.97 | ||||||
| C2 | 125.14 | 137.77 | 30.79 | 206.30 | ||||||
| T2 | 147.16 | 135.88 | 26.72 | 190.23 | ||||||
NOTE. Group specifications are as follows: C1 = Control group 1, no treatment effect, 10% constant mortality rate; T1 = Treatment group 1, 20% (20 per 100) receiving treatment effect, 10% constant mortality rate; C2 = Control group 2, no treatment effect and variable mortality by severity of AD; T2 = Treatment group 2, 20% (20 per 100) receiving treatment effect, and variable mortality by severity of AD.
The variable annual mortality rates by AD severity at 5.5% Mild, 21.5% Moderate and 48% Severe (Spackman et al [27]).
The simulated treatment effect adjusts progression in year 1 to reflect 20 persons expected to move to a worse health state remain in their starting state (i.e. 20% of participants receive a treatment effect).
We predict the expected progression for a cohort of people with mild to moderate AD (controls) and compare this with the predicted progression of AD where that same cohort, starting in the same states, are subject to a hypothetical treatment scenario in year one, with a simulated treatment effect reflected through a delay in the expected disease progression. This treatment scenario is used to simulate the potential effect of a future health intervention, providing an improvement in health status (health state location) during year one. We make a conservative assumption that 20% of the treated cohort receives a treatment effect that delays the expected (control cohort) progression of AD (i.e., people remain in the same state at year 1 to year 2, when they would otherwise be expected to move to a state with greater impairment in year 2). We compare the difference in progression of AD, controls versus the treatment scenario, in a cohort with mild to moderate AD, describing the proportion of the starting cohort in mild, moderate, and severe health states over time, and by comparing the number of years in each of these different severity states over a 5-year time horizon.
3. Results
At the time of data collection, we extracted data from the NACC UDS on 3009 participants with complete data and meeting the inclusion criteria. Most of the participants extracted had a diagnosis of probable AD on entry to the NACC data set (n = 2448, 81%). Table 3 presents the participant characteristics. As anticipated, the changes in symptom domains reflected a worsening of health status over time, with a mean (SD) decline on each domain over the initial 12-month follow-up of 2.28 (0.15) points on MMSE, 0.6 (0.10) points on the NPI-Q, and 3.8 (0.21) points on the FAQ. Most people experienced deterioration in symptom scores over months 0–12, but an improvement is reported for a significant proportion of participants, most notably for behavior and mood (NPI-Q). A worsening of scores on cognitive function, behavior and mood, and function was reported for 2043 (68%), 1378 (46%), and 2180 (72%) participants respectively; with improvements reported by 600 (20%), 1021 (34%), and 496 (16%) participants respectively. Table 2 presents the location of participants at baseline (n = 3009) across the health state descriptive system. Three health states were not populated (321, 331, and 311) and four states (231, 312, 322, 332) were unlikely to be populated (i.e., <10 participants) and were merged with adjacent (next highest) states, resulting in a set of 20 states for the model presented here. Over the 0–12 month follow-up, 2174 people (>72%) moved health state, with 835 remaining in their starting state. In those moving state, the majority (n = 1,247, 57%) moved due to a change in only one symptom domain, with 897 of these (>70%) being unrelated to the cognitive score. In addition, one third of those moving states due to multiple symptom changes were unrelated to cognitive function. Although the majority worsened on health status, a large number (n = 352, 16%) moved state due to an improvement in their health status, most commonly (220 of 352) due to reported symptoms on behavior and mood (NPI-Q).
Table 3.
Participant baseline demographic and clinical characteristics (N = 3009)*
| Age (mean, SD, range) | 75.59, 9.19, (50–104) |
| Male | 44.10% |
| Years of education (mean, SD, range) | 14.60, 6.64, (0–25) |
| Race (%) | |
| White | 82.98 |
| Black or African American | 11.67 |
| Other | 5.35 |
| Language (%) | |
| English | 92.66 |
| Spanish | 5.72 |
| Other | 1.62 |
| Living situation (%) | |
| Alone | 15.82 |
| Spouse/Partner | 66.4 |
| Relative or Friend | 12.89 |
| Other | 4.89 |
| Independence (%) | |
| Able to live independently | 21.77 |
| Requires some assistance with complex activities | 52.87 |
| Requires some assistance with basic activities | 20.31 |
| Complete dependent | 4.75 |
| Unknown | 0.3 |
| Residence (%) | |
| Single family | 87.67 |
| Retirement community | 5.52 |
| Assisted living/boarding/adult family home | 3.86 |
| Skilled nursing home | 1.13 |
| Other | 1.82 |
| Informant (%) | |
| Spouse/partner | 61.28 |
| Child | 29.43 |
| Other | 9.29 |
| MMSE (mean, SD, range) | 20.24, 5.08, (0–26) |
| NPI-Q-10 (mean, SD, range) | 3.55, 3.76, (0–26) |
| FAQ (mean, SD, range) | 15.82, 8.50, (0–30) |
We have not assessed data on AD medication use, but Spackman et al [27] report data from the NACC-UDS in a similarly defined cohort (n = 3852) where 70% of participants report taking an AD drug (cholinesterase inhibitors or memantine).
In participant data identified here, 50% (n = 1491) of participants provided data with 24-month follow-up, and 25% (n = 734) provided data with 36-month follow-up. We estimate transition probabilities using a fixed cohort approach using data over a 0–12-month follow-up from 3009 participants and do not combine data from the three groups of participants with differential follow-up (1, 2, and 3 year follow-up). Table 4 presents the transition probabilities estimated. In this table, we see that for many of the health states there is a high probability of moving to a different state over a 12-month period, some of these transitions can be to states that may be similar in terms of severity or may reflect an improvement in health status, but in many instances and especially for mild and moderate AD, the transitions are to states that reflect a worsening of health status. For example, for states 112, 121, 122, 211, and 212, between 46% and 63% of people are predicted to move to a health state the represents a clear worsening of AD severity.
Table 4.
Transition matrix for the 20 state AD progression model (transition probabilities between health states)
| To state |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From state | 111 | 112 | 113 | 121 | 122 | 123 | 131 | 132 | 133 | 211 | 212 | 213 | 221 | 222 | 223 | 232 | 233 | 313 | 323 | 333 |
| 111 | 0.38 | 0.23 | 0.02 | 0.05 | 0.07 | 0.01 | 0.01 | 0.01 | — | 0.07 | 0.09 | 0.01 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | — | — |
| 112 | 0.03 | 0.34 | 0.04 | 0.01 | 0.14 | 0.03 | — | 0.02 | 0.01 | 0.01 | 0.18 | 0.07 | 0.00 | 0.05 | 0.04 | 0.02 | 0.02 | 0.01 | 0.01 | 0.00 |
| 113 | — | 0.05 | 0.14 | — | 0.02 | 0.06 | — | 0.02 | 0.08 | — | 0.03 | 0.25 | — | — | 0.19 | 0.02 | 0.13 | — | — | 0.02 |
| 121 | 0.16 | 0.26 | 0.01 | 0.11 | 0.15 | — | 0.01 | 0.07 | — | 0.02 | 0.07 | — | 0.02 | 0.10 | — | 0.03 | — | — | — | — |
| 122 | 0.02 | 0.18 | 0.04 | 0.01 | 0.21 | 0.06 | 0.01 | 0.07 | 0.03 | — | 0.12 | 0.03 | 0.01 | 0.09 | 0.05 | 0.03 | 0.04 | 0.00 | 0.01 | 0.01 |
| 123 | — | 0.02 | 0.05 | — | 0.04 | 0.20 | — | — | 0.07 | — | 0.05 | 0.18 | — | 0.05 | 0.18 | — | 0.13 | — | 0.04 | — |
| 131 | 0.10 | 0.10 | 0.03 | 0.10 | 0.17 | — | 0.07 | 0.14 | 0.03 | 0.07 | — | — | — | 0.07 | — | 0.10 | — | — | — | — |
| 132 | — | — | 0.04 | 0.03 | 0.15 | 0.05 | 0.01 | 0.18 | 0.08 | — | 0.03 | 0.07 | — | 0.09 | 0.07 | 0.10 | 0.07 | 0.01 | — | — |
| 133 | — | — | 0.06 | — | 0.03 | 0.21 | — | 0.03 | 0.15 | — | — | — | — | — | 0.21 | 0.03 | 0.27 | — | — | — |
| 211 | 0.08 | 0.12 | 0.03 | — | 0.06 | — | — | — | — | 0.10 | 0.36 | 0.05 | 0.01 | 0.08 | 0.01 | 0.03 | 0.01 | 0.05 | — | — |
| 212 | 0.01 | 0.07 | 0.01 | — | 0.02 | 0.01 | 0.00 | 0.01 | — | 0.01 | 0.32 | 0.14 | 0.00 | 0.12 | 0.09 | 0.03 | 0.04 | 0.07 | 0.03 | 0.02 |
| 213 | — | 0.01 | 0.04 | — | — | 0.01 | — | — | 0.01 | — | 0.06 | 0.33 | — | 0.03 | 0.17 | — | 0.07 | 0.12 | 0.11 | 0.05 |
| 221 | 0.05 | 0.15 | — | — | 0.05 | — | — | — | — | 0.10 | 0.20 | — | — | 0.20 | 0.05 | 0.15 | 0.05 | — | — | — |
| 222 | 0.01 | 0.04 | 0.01 | — | 0.04 | 0.02 | — | 0.01 | 0.02 | 0.01 | 0.14 | 0.10 | — | 0.15 | 0.17 | 0.06 | 0.10 | 0.05 | 0.03 | 0.06 |
| 223 | — | — | — | — | — | 0.03 | — | — | — | — | 0.03 | 0.14 | — | 0.03 | 0.30 | 0.02 | 0.16 | 0.10 | 0.11 | 0.08 |
| 232 | — | 0.02 | 0.05 | — | 0.03 | 0.02 | — | 0.10 | 0.02 | — | — | 0.11 | 0.02 | 0.24 | 0.06 | 0.10 | 0.11 | 0.08 | 0.03 | 0.03 |
| 233 | — | — | — | — | — | 0.01 | — | — | 0.04 | — | 0.01 | — | — | 0.03 | 0.30 | 0.01 | 0.26 | 0.04 | 0.08 | 0.22 |
| 313 | — | — | — | — | — | — | — | — | — | — | 0.02 | — | — | — | 0.02 | — | 0.02 | 0.42 | 0.37 | 0.14 |
| 323 | — | — | — | — | — | — | — | — | — | — | — | 0.02 | — | — | 0.03 | — | 0.02 | 0.29 | 0.43 | 0.22 |
| 333 | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.04 | 0.04 | — | 0.04 | 0.04 | 0.25 | 0.61 |
NOTE. See Table 1 for descriptive system.
NOTE. State 231 is merged with 232, 312 with 313, 322 with 323, and 332 with 333.
Table 5 presents the predicted progression of AD over 5 years for a cohort with mild to moderate AD. When using a standardized mortality rate for AD (C1), we see that of those alive after 5 years over half are in severe AD health states. Only 5.3% of the starting cohort (69.5% start in mild states) are alive in mild AD states after 5 years, with 41% of the total cohort having died over this time. In this scenario, the cohort (controls, C1) spends 120, 179, and 90 years in mild, moderate and severe AD states, respectively, over 5 years, with mortality accounting for 111 years over that period. When predicting progression for the same cohort and when a hypothetical treatment effect is applied, retaining the assumption of a standardized mortality rate (T1), we see fewer years in the more severe states, with 140, 173, and 76 years in mild, moderate, and severe states, respectively, with mortality held constant compared to the control cohort.
Assessing a scenario with mortality rates applied by AD severity, with a much increased risk of death per year in moderate and severe states, applied to the same starting cohort (C2 and T2), we see marked differences in the comparison between controls (C2) and the hypothetical treatment cohort (T2). In this scenario, controls spend 125, 138, and 31 years in mild, moderate, and severe AD states, respectively, over 5 years, with mortality accounting for 206 years over that period. When predicting progression for the same cohort scenario, applying mortality rates by AD severity, but additionally with a hypothetical treatment effect applied, we see a marked mortality impact with the treatment effect resulting in an increase in life years lived. In this treatment scenario (T2), the cohort spend 147, 136, and 27 years in mild, moderate, and severe AD states, respectively, over 5 years, with mortality accounting for 190 years over that period, a reduction of 16 years in the impact of mortality on this cohort of 100 people over 5 years.
4. Discussion
Here, we report that many people with AD change health status, in a potentially important way, through changes in either behavior or in functional impairment, either with or without a decline in cognitive function. Prior reports of the annual rate of change on MMSE [23] and the magnitude of the annual rates of change in behavior and function [24] are consistent with those reported here. To date, the assessment of interventions, in many instances, has focused on the impacts of AD in relation to cognitive function. Results here indicate that this may be an overly narrow focus on the impacts of AD. We highlight the multidomain nature of AD and the importance of looking beyond cognitive function. Prior studies [9,23–25] have reported the fluctuating nature of symptom scores, particularly the episodic nature of behavioral symptoms, and longitudinal participant level data [24] have indicated that symptom scores for cognition, function, and behavior in people with AD can improve as well as decline, as reported in the data here.
In a health policy context, the challenge is often to compare alternative intervention strategies to estimate differences in health outcomes and costs. For AD, this requires assessment of the progression of dementia, and here, we explore how this can be done using the model presented, applying an adjustment to the transition probabilities for a “treated” cohort to impact on the predicted progression profile in that cohort, for comparison with the control cohort of people with mild to moderate AD. Similarly, a difference could be estimated based on different starting distributions across health states for a comparison of cohorts with AD. For example, using individual participant level data available at the end of a clinical trial, where a treatment has been able to alter the symptom domain scores across one or all the three symptom domains used here.
In the comparative scenario presented here, using a relatively modest although hypothetical expected intervention effect, with 20% of participants in a treatment scenario experiencing a meaningful positive effect from treatment, we report data to suggest an improvement in health status where annual mortality impact (rate) is held constant between groups. Where a potentially more realistic scenario is explored on mortality data, with increasing rates of mortality as AD progresses to more severe stages, we see an improved health status profile in the scenario with a simulated treatment effect, and we see a positive impact on the expected mortality compared to the control cohort. To put such differences into context, prior cost effectiveness analyses have reported relatively small differences in outcomes over time. For example, Bond et al. report an evaluation comparing cholinesterase inhibitors with best supportive care in mild to moderate AD, using a 20-year time horizon, finding differences of 0.03 to 0.04 quality-adjusted life years (QALYs) and a difference of 1.5 months in time to institutionalization [26].
We are aware of other promising approaches to model AD in a multidomain framework. For example, Spackman et al. [27] have reported transition probabilities for AD, by disease severity (mild, moderate, and severe) and care setting (community and institutional), using NACC-UDS data linked to the Clinical Dementia Rating (CDR) scale. The CDR is used to assess cognitive and functional performance in six areas; however, memory (cognitive function) is the primary area, and the CDR reflects the memory score unless three or more of the secondary categories score above or below the memory score [28]. In the transition probabilities presented by Spackman et al., the progression from mild to moderate AD over time is relatively slow. For example, where people are in mild AD, they have a 0.77 probability or remaining in a mild AD state over 12 months (if moderate AD, a 0.58 probability of remaining in moderate or moving to mild AD). See Supplementary Table B for a comparison of the transitions reported by Spackman et al. with those presented here. Stallard et al. [29] have presented a multidimensional model of the course of AD, using Grade of Membership (GoM) statistical techniques to consider large amounts of data on individuals with AD. The GoM model can represent multiple attributes including measures of cognitive functioning, function and behavior and mood, alongside other participant characteristics and is a promising approach. However, it appears technically challenging for analysts who require a methodology to apply based on secondary data and evidence synthesis methodology. Other than the initial application from Stallard et al. [29], to assess the impact of AD progression on costs, the GoM model has not been applied in a health policy context. Other studies have presented multidomain modeling (see Supplementary Table A), but none to date to our knowledge afford each of the symptom domains equal standing, as is the case in the model presented here.
4.1. Limitations
We develop a simple descriptive system covering the dementia stage of Alzheimer's disease. The descriptive system is based on the three main symptom domains with three levels of severity for each of the symptoms. Although the descriptive system is simple and seeks to be practical, and it still provides a set of up to 27 health states (27 × 27 transitions, in theory), it may not capture the impacts of AD in some areas. For example, where there are changes in symptom domain scores these may not lead to a change in health state, albeit evidence suggests that there are cost impacts associated with incremental changes on domains such as cognition [30] and behavior [31]. However, a more complex descriptive system is considered impractical for the currently available data.
We acknowledge that the data used are from commonly applied measurement instruments, and that there are practical and methodological concerns with many of the instrument used in AD [32] including those used here. However, we would contend that the descriptive system here may be generalizable to other judgments on severity categories when other measurement instruments are used (available) given the broad approach taken to categorizing no or mild, moderate (some), and severe problems. However, empirical investigation is required to support this assertion.
The descriptive system covers a wider range of health states (20 states) than typically seen in other models for AD; however, in the current analyses, we have reduced the descriptive system to three categories of AD severity (mild, moderate, and severe) for purposes of presentation. In the next stage of model development, we expect to estimate resource use (costs) and a summary measure of health-related quality of life (i.e., health state values, QALY weights) for each of the states. This will allow the model to be demonstrated more fully and to be tested in a health policy context, using costs and outcomes as a primary basis for comparison of interventions.
In the current presentation of the model, using simplified AD severity categories of mild, moderate, and severe, applying data estimated in Table 5, we estimate here UK costs and QALY outcomes over time (5 years), for illustrative purposes only. We have used annual cost estimates of £6300, £7704, and £11,640 for health and social care service costs for mild, moderate, and severe AD health states [3]. We have used health state values (QALY weights) of 0.71 and 0.64 for mild and moderate AD health states [3], and 0.38 for the severe AD health state [33]. We discount future costs and benefits at a rate of 3.5% per year [34,35]. In a comparison of the control and hypothetical treatment cohorts (C1 and T1, Table 5), we estimate a mean QALY gain of 0.05 over 5 years, and a saving in care costs of approximately £800 (excluding any additional treatment costs). In a comparison of cohorts where mortality rates are based on AD severity (C2 and T2, Table 5), we estimate a mean QALY gain of 0.12 and an additional care cost of £670 (excluding any additional treatment costs). This latter scenario demonstrates the perverse finding in decision analytic models of AD whereby improving mortality increases costs of care (due to the longer period lived and requiring care costs) and acts as a penalty on an effective intervention. These crude decision-analytic data are based on what may be regarded as a modest hypothetical effect, and on cost data which may not fully reflect the true costs of patients in severe states, and it is only presented here for illustrative purposes. We suggest that the illustration here does not capture the potential of the modeling framework presented here. We expect that in a future full application of the model, in a decision-analytic context, where health state costs and health state values are estimated based on the descriptive system used, analyses will be more detailed and potentially more useful in a policy context.
The NACC UDS is a large database of standardized clinical data but we acknowledge that there may be limitations linked to the generalizability of the sample used here. Sample characteristics indicate a broadly representative sample of people with a diagnosis of probable AD, but readers are advised to consider generalizability in specific applications of the model. Although we do not report specific data on the number of participants taking an AD drug (cholinesterase inhibitors or memantine), prior reports of this sample [27] indicate that most of the participants will be on AD drug treatment when data are collected. Applying core inclusion criteria here, including the availability of data on cognitive function (MMSE) over two consecutive time points resulted in a potential participant sample of n = 3316. However, the absence of data on function (FAQ) or behavior (NPI-Q) resulted in the exclusion of 307 participants, and this may have led to some bias in the data used. Although it is not possible to suggest the likely direction of that bias (i.e., a less severe or more severe participant sample), the relatively small number of exclusions may limit the impact of such bias.
We present results from the use of the transition probabilities and modeling framework over a 5-year time horizon, to demonstrate the approach, and are aware this may be a limitation in some settings given expected survival rates may be longer in some people with AD. We have therefore provided results from modeling the same mild to moderate cohort of people with AD, using different mortality assumptions (cohorts C1 and C2), over a 10 year time period, see Supplementary Table C.
The transition probabilities estimated are based on follow-up over a 0–12 month duration, and this is a potential limitation in the approach. Ideally, we would like to have a longer follow-up period, over subsequent years, but the nature of AD leads to a loss to follow-up in observational studies and to a challenge created by truncated data. We have chosen to present transit probabilities based on the observed data, across a generalized participant cohort with AD, rather than to apply statistical methods to adjust for censored data (e.g., survival analysis methods) or to stratify analyses or data by participant characteristics. Across the matrix of transit probabilities, a large data set is preferable to capture the evaluative space, and stratifying by characteristics would introduce limitations with the data (i.e. lack of coverage across the transition matrix). However, in Supplementary Table D, we present the transition probabilities estimated over an alternative scenario, using participant data “pooled” over all time periods, regardless of whether participants contribute 1, 2, or 3-year of follow-up data. Assuming time independence for transitions over a 12-month time period, this provides 5234 data points on transitions between states over a 12-month period. In this scenario, applying transit probabilities estimated from “pooled” data, which we consider the most appropriate comparison (from available data) to our primary results, we present Supplementary Table E on predicted progression of AD for comparison with data in Table 5, with results broadly similar.
A further limitation is the absence of a clear evidence base on the mortality rates for AD by disease severity. Although evidence suggests an increasing mortality risk where AD severity worsens, there is a sparsity of data to inform on mortality rates by severity. Here, we use data from a prior study reporting on the participants in the NACC UDS, which presents a steep gradient on annual mortality risk (from 5% to 48%) by AD severity [27]. There is evidence to support the high mortality rate reported here, and applied in the current analyses, from studies reporting relative risks across AD severity [36,37].
We have been unable to compare the predicted progression of AD with observed data, as a means of validating the model, and we therefore acknowledge this as a limitation with the approach presented. We have provided a simple presentation on the comparison of transition probabilities reported here, and those reported by Spackman et al [27] in Supplementary Table B, using a cohort of people with mild to moderate AD. Validation of modeling methods is a general challenge in the area of AD, and other models suffer from this limitation, given limited opportunities to access publicly available data. We hope to be able to further develop this aspect of the current research, to offer greater confidence that the modeling methods may provide a potential improvement in modeling methods for AD. At the present time, we have only been able to obtain data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) [http://www.adni-info.org/], and the data available from ADNI are for a relatively small number of participants, with participants in ADNI not commonly regarded as typical of the current clinical population of people with AD [38].
5. Conclusion
Although exploratory, the analyses presented here are able to demonstrate how a simple multidomain approach to the description of AD and the use of a simple and practical descriptive system to model the reported change in health status over time, using measures for cognitive function, behavior and mood, and functional ability, may enhance the evaluative framework for the assessment of interventions for AD. Evidence to further support the modeling framework needs to be accumulated over time.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: Health policy models used to predict the progression of Alzheimer's disease dementia have been reviewed by Green et al [3], with literature updated to 2013 here. The literature reports that cognitive function is used in most instances to predict progression of AD, and that this approach may not capture the impact of AD and/or interventions for AD.
Interpretation: The findings from our research provide empirical evidence to reinforce the importance of modeling AD over time in a multidomain manner. We report that many people with AD change health status in a meaningful way in domains other than cognition. We develop and present a multidomain health policy approach to demonstrate the value of predicting the progression of AD in this way.
Future directions: The model developed is illustrative, and further research is required to validate the approach and to test out the model in a decision analytic context.
Acknowledgments
The authors would like to thank Dr Stephen Pearson, consultant in Old Age Psychiatry, Devon Partnership Trust, UK, for his helpful review of the article, and Miss Leala Watson, University of Exeter, UK, for her help in preparation of the article. We would also like to thank four anonymous reviewers for helpful comments. No funding was received for conduct of this study. Colin Green is partly supported by the UK National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the UK NHS, the NIHR or the Department of Health.
The authors would like to thank the US National Alzheimer's Coordinating Center, and those associated with the NACC database. The NACC database is funded by the NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD).
Footnotes
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jalz.2016.01.011.
References
- 1.Prince M, Jackson J. World Alzheimer Report. Alzheimer's Disease International; London: 2009. [Google Scholar]
- 2.2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Wimo A, Reed CC, Dodel R, Belger M, Jones RW, Happich M, et al. The GERAS Study: a prospective observational study of costs and resource use in community dwellers with Alzheimer's disease in three European countries–study design and baseline findings. J Alzheimers Dis. 2013;36:385–99. doi: 10.3233/JAD-122392. [DOI] [PubMed] [Google Scholar]
- 4.Green C, Shearer J, Ritchie CW, Zajicek JP. Model-Based Economic Evaluation in Alzheimer's Disease: A Review of the Methods Available to Model Alzheimer's Disease Progression. Value Health. 2011;14:621–30. doi: 10.1016/j.jval.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JT, Neumann PJ. Decision analytic models for Alzheimer's disease: State of the art and future directions. Alzheimers Dement. 2008;4:212–22. doi: 10.1016/j.jalz.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s. Neurobiol Aging. 1998;19:173–89. doi: 10.1016/s0197-4580(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 7.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 9.Tractenberg RE, Aisen PS, Weiner MF, Cummings JL, Hancock GR. Independent contributions of neural and “higher-order” deficits to symptoms in Alzheimer's disease: A latent variable modeling approach. Alzheimers Dement. 2006;2:303–13. doi: 10.1016/j.jalz.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of Functional Activities in Older Adults in the Community. J Gerontol. 1982;37:323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 14.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 15.NICE Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease. NICE technology appraisal guidance [TA217] 2011 [Google Scholar]
- 16.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 17.Spalletta G, Musicco M, Padovani A, Rozzini L, Perri R, Fadda L, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:1026–35. doi: 10.1097/JGP.0b013e3181d6b68d. [DOI] [PubMed] [Google Scholar]
- 18.Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Making. 1994;14:52–8. doi: 10.1177/0272989X9401400107. [DOI] [PubMed] [Google Scholar]
- 19.Claus JJ, van Gool WA, Teunisse S, Walstra GJ, Kwa VI, Hijdra A, et al. Predicting survival in patients with early Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9:284–93. doi: 10.1159/000017073. [DOI] [PubMed] [Google Scholar]
- 20.Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311:33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62:779–84. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 22.Thies WH. Alzheimer's Disease Neuroimaging Initiative: A decade of progress in Alzheimer's disease. Alzheimers Dement. 2015;11:727–9. doi: 10.1016/j.jalz.2015.06.1883. [DOI] [PubMed] [Google Scholar]
- 23.Behl P, Stefurak TL, Black SE. Progress in clinical neurosciences: cognitive markers of progression in Alzheimer's disease. Can J Neurol Sci. 2005;32:140–51. doi: 10.1017/s0317167100003917. [DOI] [PubMed] [Google Scholar]
- 24.Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19:532–42. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohs RC, Schmeidler J, Aryan M. Longitudinal studies of cognitive, functional and behavioural change in patients with Alzheimer's disease. Stat Med. 2000;19:1401–9. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1401::aid-sim432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess. 2012;16:1–470. doi: 10.3310/hta16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spackman DE, Kadiyala S, Neumann PJ, Veenstra DL, Sullivan SD. Measuring Alzheimer disease progression with transition probabilities: estimates from NACC-UDS. Curr Alzheimer Res. 2012;9:1050–8. doi: 10.2174/156720512803569046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris JC. The Clinical Dementia Rating (CDR) - Current Version and Scoring Rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 29.Stallard E, Kinosian B, Zbrozek AS, Yashin AI, Glick HA, Stern Y. Estimation and Validation of a Multiattribute Model of Alzheimer Disease Progression. Med Decis Making. 2010;30:625–38. doi: 10.1177/0272989X10363479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handels RL, Wolfs CA, Aalten P, Verhey FR, Severens JL. Determinants of care costs of patients with dementia or cognitive impairment. Alzheimer Dis Assoc Disord. 2013;27:30–6. doi: 10.1097/WAD.0b013e318242da1d. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson L, Eriksdotter Jonhagen M, Kilander L, Soininen H, Hallikainen M, Waldemar G, et al. Determinants of costs of care for patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:449–59. doi: 10.1002/gps.1489. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord. 2012;5:349–58. doi: 10.1177/1756285612455733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann PJ, Kuntz KM, Leon J, Araki SS, Hermann RC, Hsu MA, et al. Health utilities in Alzheimer's disease: a cross-sectional study of patients and caregivers. Med Care. 1999;37:27–32. doi: 10.1097/00005650-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Drummond MF, Schulpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; Oxford: 2015. [Google Scholar]
- 35.National Institute for Health and Care Excellence [January 15, 2016];Guide to the methods of technology appraisal. 2013 Available at: http://www.nice.org.uk/article/pmg9/chapter/foreword. [PubMed]
- 36.Tschanz JT, Corcoran C, Skoog I, Khachaturian AS, Herrick J, Hayden KM, et al. Dementia: the leading predictor of death in a defined elderly population: the Cache County Study. Neurology. 2004;62:1156–62. doi: 10.1212/01.wnl.0000118210.12660.c2. [DOI] [PubMed] [Google Scholar]
- 37.Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R. Predictors of mortality in patients with Alzheimer's disease living in nursing homes. J Neurol Neurosurg Psychiatry. 1999;67:59–65. doi: 10.1136/jnnp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, et al. Impact of the Alzheimer's Disease Neuroimaging Initiative, 2004 to 2014. Alzheimers Dement. 2015;11:865–84. doi: 10.1016/j.jalz.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.