Abstract
Objective
To develop and validate a tool to predict the risk of all-cause readmission within 30 days (30-d readmission) among hospitalized patients with diabetes.
Methods
A cohort of 44,203 discharges was retrospectively selected from the electronic records of adult patients with diabetes hospitalized at an urban academic medical center. Discharges of 60% of the patients (n = 26,402) were randomly selected as a training sample to develop the index. The remaining 40% (n = 17,801) were selected as a validation sample. Multivariable logistic regression with generalized estimating equations was used to develop the Diabetes Early Readmission Risk Indicator (DERRI™).
Results
Ten statistically significant predictors were identified: employment status; living within 5 miles of the hospital; preadmission insulin use; burden of macrovascular diabetes complications; admission serum hematocrit, creatinine, and sodium; having a hospital discharge within 90 days before admission; most recent discharge status up to 1 year before admission; and a diagnosis of anemia. Discrimination of the model was acceptable (C statistic 0.70), and calibration was good. Characteristics of the validation and training samples were similar. Performance of the DERRI™ in the validation sample was essentially unchanged (C statistic 0.69). Mean predicted 30-d readmission risks were also similar between the training and validation samples (39.3% and 38.7% in the highest quintiles).
Conclusion
The DERRI™ was found to be a valid tool to predict all-cause 30-d readmission risk of individual patients with diabetes. The identification of high-risk patients may encourage the use of interventions targeting those at greatest risk, potentially leading to better outcomes and lower healthcare costs.
INTRODUCTION
Hospital readmissions within 30 days of discharge (30-d readmissions) have become a high-priority healthcare quality measure and target for cost reduction (1-3). In 2012, hospital care for patients with diabetes cost approximately $124 billion in the U.S. (4). Although individuals with diabetes represent about 9% of the U.S. population (5), they account for nearly 25% of hospitalizations each year (6). The reported 30-d readmission rate of patients with diabetes is as high as 21.0% (7-12), corresponding to nearly 2 million discharges annually (6).
Although modestly effective interventions to reduce the risk of 30-d readmission of various populations have been reported (13), specific approaches are needed for patients with diabetes (14). Interventions designed to reduce 30-d readmission risk among patients with chronic disease have not consistently been shown to be effective when applied on a large scale (13,15), and general application would be cost-prohibitive in most healthcare systems. If high-risk patients could be identified, then interventions could be targeted to those at greatest risk of readmission, enabling more efficient resource use. Therefore, an accurate method to identify patients with diabetes at high risk for 30-d readmission is required. Most studies of readmission risk factors among patients with diabetes have been limited by the use of administrative rather than clinical data, lack of applicability at the point of care, and/or a narrow focus on a primary discharge diagnosis of diabetes (7,8,16-19). Furthermore, there is no existing readmission risk prediction tool specifically for diabetes patients.
We therefore developed and validated a model to predict the risk of all-cause 30-d readmission in hospitalized patients with diabetes, the Diabetes Early Readmission Risk Indicator (DERRI™), based on easily obtained clinical and sociodemographic information available before hospital discharge.
METHODS
Study Sample
A cohort of 44,203 discharges was retrospectively selected from the electronic medical records of 17,284 patients hospitalized at an urban academic medical center (Boston Medical Center, Boston, MA) between January 1, 2004 and December 31, 2012, the time period for which data were available. Inclusion criteria were a diagnosis of diabetes defined by an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 250.xx associated with hospital discharge or the presence of a diabetes-specific medication on the preadmission medication list. Index discharges were excluded for patients younger than 18 years, discharge by transfer to another hospital, discharge from an obstetric service (indicating pregnancy), inpatient death, outpatient death within 30 days of discharge, incomplete data or lacking 30 days of follow up after discharge (discharged after December 1, 2012). Readmission that occurred within 8 hours of an index discharge was considered a false positive and merged with the discharge to avoid counting in-hospital transfer as a readmission. All eligible discharges were included in the analysis.
The cohort was divided randomly into training and validation samples (20). The training sample, which comprised 60% of the patients in the study cohort, was used to develop the DERRI™. The validation sample contained the remaining 40% of the cohort and was used to test DERRI™ performance. The modest deviation from the commonly used 2:1 ratio of dividing a sample into training and validation sets was to optimize the sample size of the smaller validation set.
For the study duration, discharge procedures were conducted by the primary nurse in a standard fashion, which included medication reconciliation and basic education, as well as insulin teaching when needed. Glucose meter teaching was available for inpatients by a diabetes educator, which was in place before 2004. There were no specific diabetes-focused interventions for discharging patients.
The Boston Medical Center and Temple University Institutional Review Boards approved the protocol.
Definition of Variables
The outcome to be predicted by the model was all-cause readmission within 30 days of the index discharge. Forty-six variables were evaluated as predictors of the outcome. Most variables were based on information obtained during the index hospitalization. For all but 1 of the laboratory parameters (serum albumin), the first value available during the time period starting 24 hours before the time of admission was used. This sampling allowed for inclusion of values obtained in the immediate preadmission time period (usually obtained in the emergency department). For serum albumin, the value closest to the date and time of admission was used up to 30 days before or during the admission. For weight, the first value obtained during the index hospitalization or, if unavailable, the value closest to the date and time of admission was used up to 1 year before admission. Missing weights (9,680 discharges) were imputed based on height, age, race, and sex. Missing heights (3,481 discharges) were imputed based on age, race, and sex. Variables based on ICD-9-CM codes were considered for ever occurrence (during or before the index hospitalization) or current occurrence during the index hospitalization (Table 1). No variables were based on summary statistics of laboratory values or combinations of diagnostic codes to maximize ease of use at the point of care in the future. The most common reasons for 30-d readmission based on primary ICD-9-CM code were described.
Table 1. Definition of Variables based on ICD-9-CM Diagnosis Codes.
| Variable | Category (ICD-9-CM code) |
|---|---|
| Current or prior DKA or HHS |
Yes (250.1x or 250.2x), no |
| Microvascular complicationsa |
Number of diagnoses (362.0x,
250.6x, or 250.4x) up to 3 |
| Macrovascular complicationsb |
Number of diagnoses
(410.xx-414.xx; 428.xx; 434.xx, 435.x, 437.1, 438.xx, or 997.02; 250.7x, 440.xx, 443.xx, or 444.xx) up to 4 |
| Schizophrenia or mood disorder |
Yes (295.xx or 296.xx), no |
| Gastroparesis | Yes (536.3), no |
| Pancreatitis | Yes (577.0 or 577.1), no |
| Hypertension | Yes (401.x or 405.xx), no |
| COPD or asthma | Yes (491.2x or 493.xx), no |
| Cardiac dysrhythmia | Yes (427.xx), no |
| Malignant neoplasm | Yes (140.x-165.x, 170.x-176.x,
179, 180.x-199.x, or 200.xx-202.xx), no |
| Anemia | Yes (280.xx-285.xx), no |
| Drug abuse | Never, prior (negative or no
drug screen and 304.xx or 305.xx), current (positive drug screen and 304.xx or 305.xx) |
| Infectionc | Yes (480.xx-486.xx, 595.0, 599.0,
038. xx, 681.xx, 682.x, or 686.xx), no |
| Complication of device, graft, or implant |
Yes (996.0x-996.7x), no |
| Fluid or electrolyte disorder |
Yes (276.xx), no |
Abbreviations: COPD = chronic obstructive pulmonary disease; DKA = diabetic ketoacidosis; HHS = hyperosmolar hyperglycemic state; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; No = not recorded.
Retinopathy, neuropathy, nephropathy
Coronary artery disease, heart failure, stroke, peripheral vascular disease
Pneumonia, urinary tract infection, septicemia, skin or subcutaneous infection
Statistical Analysis
Summaries of categorical variables included counts and percentages, while means and SDs or medians and interquartile ranges were used for continuous variables. Readmitted patients were compared to nonreadmitted patients by χ2 tests for categorical variables and 2-sample t tests or Wilcoxon rank sum tests for continuous variables. Nonnormally distributed continuous variables were log transformed for modeling procedures. The generalized estimating equations (GEE) approach was used to model the association of the predictors with 30-d readmission (21). In contrast to logistic regression without GEE, which assumes independence of each observation, the GEE method accounts for clustering of repeat observations, in this case, multiple discharges per patient. The initial model included all the variables associated with 30-d readmission in univariate analyses in the training sample (P<.01). Multivariable logistic regression with GEE was performed to determine the adjusted associations of the variables with all-cause 30-d readmission. The most parsimonious model that optimized predictive performance was selected as the final DERRI™ model. Ease of use at the point of care and collinearity were considered in developing the model.
Assessment of model performance was based on discrimination (the ability of the model to distinguish between high- and low-risk individuals) and calibration (the ability of the model to correctly estimate risk across the range of potential risk) (20,22). Discrimination was evaluated using the C statistic, which represents the area under the receiver operating characteristic (ROC) curve (23), where higher values represent better discrimination (24). Calibration was assessed by the Hosmer-Lemeshow test, where P>.05 indicates adequate calibration (20). Using the DERRI™ to predict each patient’s risk of readmission as a number between 0 and 100%, patients were stratified into quintiles of 30-d readmission risk. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). P<.05 was considered statistically significant.
RESULTS
There were 44,203 discharges in the entire sample, of which 9,034 (20.4%) were associated with 30-d readmission for any cause. Characteristics of the training sample (n = 26,402 discharges) are presented in Table 2. The sample was well distributed across middle to older adult ages, racial/ethnic backgrounds, types of health insurance, educational levels, and employment status. About half were female, two-thirds lived within 5 miles of the hospital, a majority was overweight or obese, and a substantial minority (19%) did not speak English. Regarding preadmission diabetes therapies, 28% of discharges were among patients treated with metformin, 15% were treated with a sulfonylurea, and 37% used insulin. At least 1 microvascular complication was documented for 30% of discharges, whereas 56% had ≥1 macrovascular complication. The most common nondiabetes-related comorbidities were hypertension, anemia, and depression or psychosis. Most of the variables were associated with 30-d readmission in univariate analysis (Table 2). Sex, English fluency, preadmission sulfonylurea use, hypertension, age, inpatient diabetes consultation, and drug abuse were not associated with 30-d readmission. The relatively large minority of non-English speakers (mostly Spanish and Haitian Creole) provided ample data to have confidence in this finding. Common primary diagnoses for 30-d readmission were diabetes; heart failure; shortness of breath; chest pain; acute kidney failure; complication or infection of a device, implant, graft, or indwelling urinary catheter; and postoperative complications (Table 3). The most frequent complications occurred with a vascular (including renal dialysis), cardiac, or orthopedic device, implant, or graft.
Table 2. Characteristics of Hospitalized Patients with Diabetes in the Training Sample by 30-d Readmission Status.
| Variable | All discharges n = 26,402 |
Followed by readmission n = 5,413 |
No readmission n = 20,989 |
P |
|---|---|---|---|---|
| Age, n (%) | ||||
| <50 years | 5,152 (19.5) | 1,118 (20.7) | 4,034 (19.2) | .72 |
| 50-59 years | 5,997 (22.7) | 1,280 (23.7) | 4,717 (22.5) | |
| 60-69 years | 6,945 (26.3) | 1,420 (26.2) | 5,525 (26.3) | |
| 70+ years | 8,308 (31.5) | 1,595 (29.5) | 6,713 (32.0) | |
| Sex, n (%) | ||||
| Female | 13,275 (50.3) | 2,600 (48.0) | 10,675 (50.9) | .79 |
| Male | 13,127 (49.7) | 2,813 (52.0) | 10,314 (49.1) | |
| Marital status, n (%) | ||||
| Married | 8,064 (30.5) | 1,502 (27.8) | 6,562 (31.3) | <.001 |
| Single | 17,784 (67.4) | 3,842 (71.0) | 13,942 (66.4) | |
| Other or not recorded | 554 (2.1) | 69 (1.3) | 485 (2.3) | |
| Race/ethnicity, n (%) | ||||
| Black | 9,694 (36.7) | 2,135 (39.4) | 7,559 (36.0) | <.001 |
| Hispanic | 3,308 (12.5) | 638 (11.8) | 2,670 (12.7) | |
| White | 6,923 (26.2) | 1,254 (23.2) | 5,669 (27.0) | |
| Other | 1,185 (4.5) | 159 (2.9) | 1,026 (4.9) | |
| Not recorded | 5,292 (20.0) | 1,227 (22.7) | 4,065 (19.4) | |
| English speaking, n (%) | ||||
| Yes | 21,487 (81.4) | 4,513 (83.4) | 16,974 (80.9) | .06 |
| No | 4,915 (18.6) | 900 (16.6) | 4,015 (19.1) | |
| Insurance status, n (%) | ||||
| Medicaid | 4,257 (16.1) | 1,003 (18.5) | 3,254 (15.5) | <.001 |
| Medicare | 10,733 (40.7) | 2,256 (41.7) | 8,477 (40.4) | |
| None | 996 (3.8) | 92 (1.7) | 904 (4.3) | |
| Private | 5,124 (19.4) | 835 (15.4) | 4,289 (20.4) | |
| Not recorded | 5,292 (20.0) | 1,227 (22.7) | 4,065 (19.4) | |
| Home zip code, n (%) | ||||
| ≥5 miles from hospital | 8,168 (30.9) | 1,309 (24.2) | 6,859 (32.7) | <.001 |
| <5 miles from hospital | 18,234 (69.1) | 4,104 (75.8) | 14,130 (67.3) | |
| Educational level, n (%) | ||||
| Less than high school | 3,518 (13.3) | 750 (13.9) | 2,768 (13.2) | <.001 |
| Any high school | 14,672 (55.6) | 3,370 (62.3) | 11,302 (53.9) | |
| Some college | 1,828 (6.9) | 380 (7.0) | 1,448 (6.9) | |
| College graduate | 3,920 (14.9) | 652 (12.1) | 3,268 (15.6) | |
| Not recorded | 2,464 (9.3) | 261 (4.8) | 2,203 (10.5) | |
| Employment, n (%) | ||||
| Disabled | 5,822 (22.1) | 1,696 (31.3) | 4,126 (19.7) | <.001 |
| Employed | 2,571 (9.7) | 267 (4.9) | 2,304 (11.0) | |
| Retired | 9,995 (37.9) | 2,030 (37.5) | 7,965 (38.0) | |
| Unemployed | 7,227 (27.4) | 1,347 (24.9) | 5,880 (28.0 | |
| Other or not recorded | 787 (3.0) | 73 (1.4) | 714 (3.4) | |
| Preadmission sulfonylurea use, n (%) | ||||
| Yes | 3,839 (14.5) | 776 (14.3) | 3,063 (14.6) | .12 |
| No | 22,563 (85.5) | 4,637 (85.7) | 17,926 (85.4) | |
| Preadmission metformin use, n (%) | ||||
| Yes | 7,387 (28.0) | 1,198 (22.1) | 6,189 (29.5) | <.001 |
| No | 19,015 (72.0) | 4,215 (77.9) | 14,800 (70.5) | |
| Preadmission thiazolidinedione use, n (%) | ||||
| Yes | 1,765 (6.7) | 267 (4.9) | 1,498 (7.1) | <.001 |
| No | 24,637 (93.3) | 5,146 (95.1) | 19,491 (92.9) | |
| Preadmission insulin use, n (%) | ||||
| Yes | 10,024 (38.0) | 2,716 (50.2) | 7,308 (34.8) | <.001 |
| No | 16,378 (62.0) | 2,697 (49.8) | 13,681 (65.2) | |
| Preadmission glucocorticoid use, n (%) | ||||
| Yes | 2,641 (10.0) | 742 (13.7) | 1,899 (9.1) | <.001 |
| No | 23,761 (90.0) | 4,671 (86.3) | 19,090 (91.0) | |
| Most extreme blood glucose level, n (%) | ||||
| 40-69 or 181-300 mg/dL | 11,582 (43.9) | 2,476 (45.7) | 9,106 (43.4) | <.001 |
| 70-180 mg/dL | 9,479 (35.9) | 1,667 (30.8) | 7,812 (37.2) | |
| <40 or >300 mg/dL | 5,341 (20.2) | 1,270 (23.5) | 4,071 (19.4) | |
| Diabetes inpatient consultation, n (%) | ||||
| Yes | 3,411 (12.9) | 612 (11.3) | 2,799 (13.3) | .82 |
| No | 22,991 (87.1 | 4,801 (88.7) | 18,190 (86.7) | |
| Current or prior DKA or HHS, n (%) | ||||
| Yes | 1,989 (7.5) | 556 (10.3) | 1,433 (6.8) | .008 |
| No | 24,413 (92.5) | 4,857 (89.7) | 19,556 (93.2) | |
| Microvascular complications,a n (%) | ||||
| 0 | 18,488 (70.0) | 3,163 (58.4) | 15,325 (73.0) | <.001 |
| 1 | 4,873 (18.5) | 1,218 (22.5) | 3,655 (17.4) | |
| 2 | 1,917 (7.3) | 612 (11.3) | 1,305 (6.2) | |
| 3 | 1,124 (4.3) | 420 (7.8) | 704 (3.4) | |
| Macrovascular complications,b n (%) | ||||
| 0 | 11,561 (43.8) | 1,892 (35.0) | 9,669 (46.1) | <.001 |
| 1 | 7,488 (28.4) | 1,521 (28.1) | 5,967 (28.4) | |
| 2 | 5,281 (20.0) | 1,306 (24.1) | 3,975 (18.9) | |
| 3 | 1,595 (6.0) | 522 (9.6) | 1,073 (5.1) | |
| 4 | 477 (1.8) | 172 (3.2) | 305 (1.5) | |
| Preadmission BP meds, n (%) | ||||
| None | 7,325 (27.7) | 1,105 (20.4) | 6,220 (29.6) | <.001 |
| ACE-i or ARB | 12,757 (48.3) | 2,795 (51.6) | 9,962 (47.5) | |
| Non-ACE or ARB | 6,320 (23.9) | 1,513 (28.0) | 4,807 (22.9) | |
| Preadmission statin use, n (%) | ||||
| Yes | 12,582 (47.7) | 2,679 (49.5) | 9,903 (47.2) | .034 |
| No | 13,820 (52.3) | 2,734 (50.5) | 11,086 (52.8) | |
| White blood cell count, n (%) | ||||
| Low <4k/μL | 1,226 (4.6) | 372 (6.9) | 854 (4.1) | .001 |
| Normal 4-11k/μL | 20,232 (76.6) | 3,977 (73.5) | 16,255 (77.5) | |
| High >11k/μL | 4,944 (18.7) | 1,064 (19.7) | 3,880 (18.5) | |
| Serum hematocrit (%), mean (SD) | 33.6 (5.25) | 34.0 (5.24) | 32.4 (5.07) | <.001 |
| Serum albumin, N (%) | ||||
| 4+ g/dL | 8,913 (33.8) | 1,602 (29.6) | 7,311 (34.8) | <.001 |
| <4 g/dL | 14,135 (53.5) | 3,330 (61.5) | 10,805 (51.5) | |
| Not recorded | 3,354 (12.7) | 481 (8.9) | 2,873 (13.7) | |
| Serum sodium, n (%) | ||||
| Low <135 mmol/L | 2,730 (10.3) | 733 (13.5) | 1,997 (9.5) | <.001 |
| Normal 135-145 mmol/L | 23,431 (88.8) | 4,621 (85.4) | 18,810 (89.6) | |
| High >145 mmol/L | 241 (0.9) | 59 (1.1) | 182 (0.9) | |
| Serum potassium, n (%) | ||||
| Low <3.1 mmol/L | 302 (1.1) | 60 (1.1) | 242 (1.2) | <.001 |
| Normal 3.1-5.3 mmol/L | 24,043 (91.1) | 4,777 (88.3) | 19,266 (91.8) | |
| High >5.3 mmol/L | 2,057 (7.8) | 576 (10.6) | 1,481 (7.1) | |
| Serum creatinine (mg/dL), median (IQR) | 1.0 (0.7-1.4) | 0.9 (0.7-1.3) | 1.1 (0.6-2.0) | <.001 |
| Body mass index, n (%) | ||||
| <18.5 kg/m2 | 616 (2.3) | 150 (2.8) | 466 (2.2) | .006 |
| 18.5-24.9 kg/m2 | 4,405 (16.7) | 960 (17.7) | 3,445 (16.4) | |
| 25.0-29.9 kg/m2 | 7,452 (28.2) | 1,503 (27.8) | 5,949 (28.3) | |
| ≥30.0 kg/m2 | 13,929 (52.8) | 2,800 (51.7) | 11,129 (53.0) | |
| Discharged 90 days prior to index admission, n (%) | ||||
| Yes | 8,507 (32.2) | 2,843 (52.5) | 5,664 (27.0) | <.001 |
| No | 17,895 (67.8) | 2,570 (47.5) | 15,325 (73.0) | |
| Discharge 1 year prior to index admission, n (%) | ||||
| Home | 9,379 (35.5) | 2,353 (43.5) | 7,026 (33.5) | <.001 |
| Home with nursing care | 3,430 (13.0) | 925 (17.1) | 2,505 (11.9) | |
| Subacute facility | 3,238 (12.3) | 896 (16.6) | 2,342 (11.2) | |
| Against medical advice | 384 (1.5) | 140 (2.6) | 244 (1.2) | |
| No discharge recorded | 9,971 (37.8) | 1,099 (20.3) | 8,872 (42.3) | |
| Urgent or emergent admission,c n (%) | ||||
| Yes | 22,780 (86.3) | 4,916 (90.8) | 17,864 (85.1) | <.001 |
| No | 3,622 (13.7) | 497 (9.2) | 3,125 (14.9) | |
| Intensive care admission, n (%) | ||||
| Yes | 4,265 (16.2) | 879 (16.2) | 3,386 (16.1) | .042 |
| No | 22,137 (83.9) | 4,534 (83.8) | 17,603 (83.9) | |
| Blood transfusion given, n (%) | ||||
| Yes | 3,610 (13.7) | 871 (16.1) | 2,739 (13.1) | <.001 |
| No | 22,792 (86.3) | 4,542 (83.9) | 18,250 (87.0) | |
| Parenteral or enteral nutrition, n (%) | ||||
| Yes | 927 (3.5) | 259 (4.8) | 668 (3.2) | .001 |
| No | 25,475 (96.5) | 5,154 (95.2) | 20,321 (96.8 | |
| Depression or psychosis ever, n (%) | ||||
| Yes | 7,870 (29.8) | 2,057 (38.0) | 5,813 (27.7) | <.001 |
| No | 18,532 (70.2) | 3,356 (62.0) | 15,176 (72.3) | |
| Gastroparesis ever, n (%) | ||||
| Yes | 1,196 (4.5) | 424 (7.8) | 772 (3.7) | <.001 |
| No | 25,206 (95.5) | 4,989 (92.2) | 20,217 (96.3) | |
| Pancreatitis ever, n (%) | ||||
| Yes | 1,413 (5.4) | 453 (8.4) | 960 (4.6) | .007 |
| No | 24,989 (94.7) | 4,960 (91.6) | 20,029 (95.4) | |
| Hypertension ever, n (%) | ||||
| Yes | 19,290 (73.1) | 3,957 (73.1) | 15,333 (73.1) | .38 |
| No | 7,112 (26.9) | 1,456 (26.9) | 5,656 (27.0) | |
| COPD or asthma ever, n (%) | ||||
| Yes | 6,365 (24.1) | 1,592 (29.4) | 4,773 (22.7) | <.001 |
| No | 20,037 (75.9) | 3,821 (70.6) | 16,216 (77.3) | |
| Cardiac dysrhythmias ever, n (%) | ||||
| Yes | 6,382 (24.2) | 1,614 (29.8) | 4,768 (22.7) | <.001 |
| No | 20,020 (75.8) | 3,799 (70.2) | 16,221 (77.3) | |
| Malignant neoplasm ever, n (%) | ||||
| Yes | 2,688 (10.2) | 715 (13.2) | 1,973 (9.4) | <.001 |
| No | 23,714 (89.8) | 4,698 (86.8) | 19,016 (90.6) | |
| Anemia ever, n (%) | ||||
| Yes | 10,916 (41.4) | 3,102 (57.3) | 7,814 (37.2) | <.001 |
| No | 15,486 (58.7) | 2,311 (42.7) | 13,175 (62.8) | |
| Drug abuse, n (%) | ||||
| Never | 21,120 (80.0) | 4,257 (78.6) | 16,863 (80.3) | .12 |
| History | 4,177 (15.8) | 897 (16.6) | 3,280 (15.6) | |
| Current | 1,105 (4.2) | 259 (4.8) | 846 (4.0) | |
| Current infection,d n (%) | ||||
| Yes | 5,989 (22.7) | 1,316 (24.3) | 4,673 (22.3) | .003 |
| No | 20,413 (77.3) | 4,097 (75.7) | 16,316 (77.7) | |
| Current complication of device, graft, or implant, n (%) | ||||
| Yes | 1,052 (4.0) | 293 (5.4) | 759 (3.6) | <.001 |
| No | 25,350 (96.0) | 5,120 (94.6) | 20,230 (96.4) | |
| Current fluid or electrolyte disorder, n (%) | ||||
| Yes | 5,351 (20.3) | 1,324 (24.5) | 4,027 (19.2) | <.001 |
| No | 21,051 (79.7) | 4,089 (75.5) | 16,962 (80.8) | |
Abbreviations: ACE = angiotensin-converting enzyme; ACE-i = ACE inhibitor; ARB = angiotensin II receptor blocker; BP = blood pressure; COPD = chronic obstructive pulmonary disease; DKA = diabetic ketoacidosis; Ever = current or prior; HHS = hyperosmolar hyperglycemic state; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; IQR = interquartile range.
Retinopathy, neuropathy, nephropathy
Coronary artery disease, heart failure, stroke, peripheral vascular disease
Emergent admission = patient required immediate intervention as a result of a severe, life threatening or potentially disabling condition. Generally, the patient was admitted through the emergency room. Urgent admission = patient required immediate attention for a less severe condition. Generally, the patient was admitted directly to the first available, suitable accommodation.
Pneumonia, urinary tract infection, septicemia, skin or subcutaneous infection
Table 3. Most Common Readmission Reasons in the Training and Validation Samples Based on Primary ICD-9-CM Code.
| ICD-9-CM | Training sample | Validation sample | |
|---|---|---|---|
| Code | Description | n, % of readmissions | n, % of readmissions |
| 250.xx | Diabetes mellitus | 461, 8.5 | 331, 9.1 |
| 428.xx | Heart failure | 446, 8.2 | 344, 9.5 |
| 996.xx | Complication or infection of device, implant,
graft, or indwelling urinary cathetera |
204, 3.8 | 140, 3.9 |
| 786.5x or 786.05 |
Chest pain or shortness of breath | 200, 3.7 | 110, 3.0 |
| 584.xx | Acute kidney failure | 185, 3.4 | 124, 3.4 |
| 998.xx | Postoperative complication, including
infection, bleeding, and disruption of surgical woundb |
162, 3.0 | 117, 3.2 |
| 599.0 | Urinary tract infection | 110, 2.0 | 82, 2.3 |
Abbreviations: ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Excludes 996.81, complications of transplanted kidney, which was ≤0.3% of readmissions
Excludes 998.89, other postoperative or blood transfusion complications, which was ≤0.1% of readmissions
The DERRI™ is composed of 10 highly statistically significant predictors selected from the 11 most significant predictors (Table 4). Patients who were discharged within 90 days before the index admission were at nearly twofold greater odds of having a 30-d readmission than patients without a recent prior discharge. Compared to patients discharged home in the year before the index admission, those without a prior discharge had a 33% lower risk of being readmitted, whereas patients previously discharged against medical advice were 49% more likely to be readmitted. Retired, unemployed, or disabled patients were at greater odds of readmission than patients who were employed. Patients with a higher admission serum creatinine or low serum sodium were at higher odds of readmission, whereas those with higher hematocrit had lower odds of readmission. Other predictors of 30-d readmission in the DERRI™ were living within 5 miles of the hospital, increasing burden of macrovascular complications of diabetes, preadmission insulin use, and a current or prior diagnosis of anemia. The C statistic was 0.70, and the Hosmer-Lemeshow test for calibration was nonsignificant (P = .39).
Table 4. DERRI™ Predictors of All-Cause 30-d Readmission in the Training Sample.
| Predictor | OR (95% CI) | P |
|---|---|---|
| Home zip code <5 miles from hospital | 1.22 (1.11-1.33) | <.0001 |
| Employment status (vs. employed) | ||
| Disabled | 1.94 (1.63-2.32) | <.0001 |
| Retired | 1.44 (1.22-1.69) | <.0001 |
| Unemployed | 1.52 (1.28-1.80) | <.0001 |
| Preadmission insulin use | 1.25 (1.14-1.36) | <.0001 |
| Macrovascular complicationsa, n (vs. 0) | ||
| 1 | 1.09 (0.97-1.21) | .14 |
| 2 | 1.15 (1.02-1.29) | .027 |
| 3 | 1.37 (1.17-1.61) | <.0001 |
| 4 | 1.43 (1.04-1.96) | .026 |
| Admission serum hematocrit, per 5% | 0.85 (0.82-0.88) | <.0001 |
| Log (admission serum creatinine) | 1.14 (1.07-1.22) | <.0001 |
| Admission serum sodium (vs. normal) | ||
| Low, <135 mmol/L | 1.32 (1.18-1.47) | <.0001 |
| High, >145 mmol/L | 1.17 (0.86-1.60) | .31 |
| Discharged within 90 d before admission | 1.93 (1.76-2.11) | <.0001 |
| Most recent discharge status up to 1 year before admission (vs. home) | ||
| Against medical advice | 1.49 (1.05-2.10) | .024 |
| Home with nursing care | 0.95 (0.85-1.06) | .34 |
| No discharge recorded | 0.77 (0.70-0.85) | <.0001 |
| Subacute facility | 0.92 (0.82-1.02) | .10 |
| Anemia, current or prior diagnosis | 1.26 (1.15-1.39) | <.0001 |
Abbreviations: CI = confidence interval; DERRI = Diabetes Early Readmission Risk Indicator; OR = odds ratio.
Coronary artery disease, heart failure, stroke, peripheral vascular disease
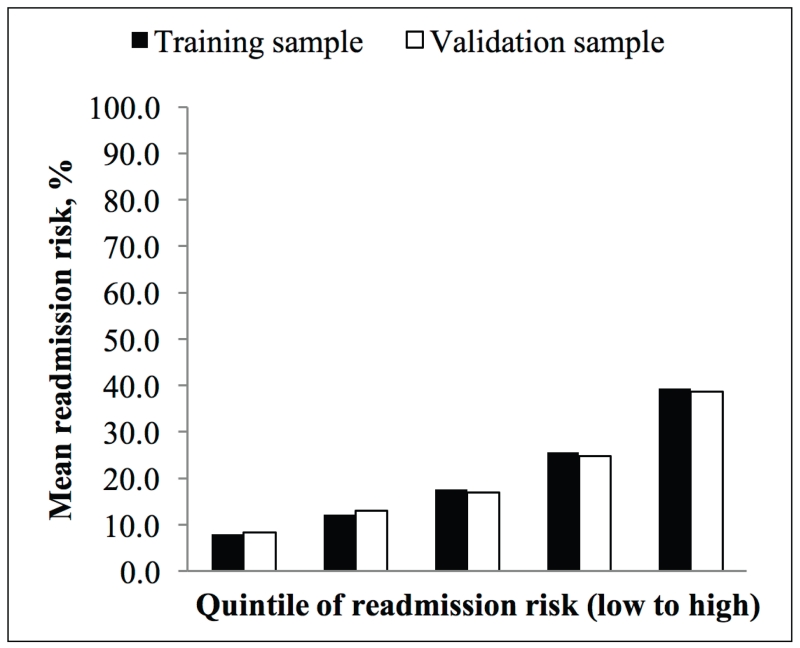
Using the DERRI™, the training sample was stratified into quintiles of predicted all-cause 30-d readmission risk (Fig. 1). The highest quintile had a 39.3% mean predicted risk of 30-d readmission and accounted for 38.4% of 30-d readmissions.
Fig. 1.
Quintiles of all-cause 30-d readmission risk predicted by the Diabetes Early Readmission Risk Indicator™ in training and validation samples.
The validation sample included 17,801 discharges. Characteristics of the validation and training samples were similar for all variables (data not shown). Only 2 variables, admission serum sodium and preadmission sulfonylurea use, displayed statistically significant differences (P = .03 for both); however, the absolute differences among the categories were <2%. Discrimination and calibration of the DERRI™ in the validation sample were essentially unchanged (C statistic 0.69, Hosmer-Lemeshow test P = .22). The predicted 30-d readmission risks were also similar between the training and validation samples (Fig. 1).
DISCUSSION
In this retrospective study of 44,203 discharges of patients with diabetes, numerous patient characteristics were associated with 30-d readmission. From these characteristics we developed a set of 10 highly statistically significant predictors of 30-d readmission to form the DERRI™. This novel predictive model successfully stratified patients into quintiles of 30-d readmission risk, and the highest quintile had an almost 40% risk of 30-d readmission. The model showed acceptable discrimination and calibration in both the training and validation samples. This tool may be useful for predicting the 30-d readmission risk of individual patients.
The reported all-cause 30-d readmission rate of patients with diabetes ranges from 10.0 to 21.0% (7-12). In our cohort, the 30-d readmission rate was 20.4%. It is important to note that our sample was drawn from an urban, academic medical center. The studies reporting lower readmission risk tended to be performed in nonurban settings (8,10,11). It is likely that urban populations have a higher risk of readmission than nonurban populations (8).
The most common reasons for readmission according to primary discharge diagnoses were diabetes, heart failure, procedural complications, chest pain, shortness of breath, acute kidney failure, and urinary tract infection. To our knowledge, only one other study has presented primary diagnoses of 30-d readmissions among diabetes patients, also reporting diabetes, renal disease, heart failure, and ischemic heart disease (25). Unlike our study, however, the report by Jiang et al was restricted to readmissions for diabetes-related conditions.
Herein, we report on a number of predictors for 30-d readmission, many of which are novel. Although discharge within 90 days prior to admission has not specifically been reported by other groups, prior hospitalizations and emergency department visits have been shown to predict 30-d readmission risk (7,10,26). Likewise, diabetes complications; preadmission insulin use; and admission serum hematocrit, sodium, and creatinine have not been previously identified as predictors of 30-d readmission, but several other groups have demonstrated that comorbidity burden is associated with readmission risk (7,8,10,26,27). In contrast, anemia has been previously reported (28).
We found that patients who lived within 5 miles of the hospital were more likely to be readmitted than patients who lived farther away. In contrast, a single-center study conducted at Ohio State University reported that home distance from the hospital was not related to readmission risk (11). This contrast may reflect differences in local healthcare infrastructure. Patients in our study who lived farther from the study hospital were often closer to other academic or nonacademic medical centers in the greater Boston area and may have been more likely to be readmitted at a closer institution. This may not have been the case near Ohio State, which has a lower hospital density.
The DERRI™ has important characteristics relative to other models of 30-d readmission. First, the C statistic of 0.70 is comparable to the C statistic reported with other models developed for diabetes patients (8,10,26). Likewise, a systematic review identified 7 readmission risk prediction models not restricted to diabetes that could be used to identify high-risk patients during a hospitalization, with C statistics ranging from 0.56 to 0.72 (29). Second, unlike other readmission models of patients with diabetes, all of the predictors included in the DERRI™ are easily obtained at the time of admission by brief patient interview and application of routinely collected clinical information. Thus, the DERRI™ could be used to identify patients at higher risk of 30-d readmission early during the course of a hospitalization, such that interventions to reduce readmission risk could be initiated before discharge. It should be noted that using information available only before discharge precludes the use of potentially important predictors such as length-of-stay and outpatient follow-up. It is our belief, however, that the ability to predict readmission risk and implement preventive strategies before discharge may trump the potential added predictive power of postdischarge information. We envision the development of an electronic readmission risk prediction tool that could be used at the point of care similar to the American College of Cardiology/American Heart Association CV Risk Calculator (30).
A number of limitations of our data should be acknowledged. This was a single-center study conducted at an urban academic medical center, and the DERRI™ may not be generalizable to other settings. Because the study was retrospective and some data were unavailable, certain potential readmission predictors of interest could not be examined, including hemoglobin A1c, diabetes type, and diabetes duration. Additional potential predictors such as poor health literacy and social determinants of health may be related to 30-d readmissions among patients with diabetes, but these factors were not available in this sample (31). It is possible that more direct measures of health literacy and socioeconomic status would add predictive power to the model. Lastly, 30-d readmissions that may have occurred at other hospitals were not captured. It seems unlikely, however, that a significant number of patients were readmitted elsewhere because the 30-d readmission rate in our study is on the higher end of the range reported in the literature for patients with diabetes.
These limitations are balanced by several strengths of the present study, including a relatively large sample size drawn from patients hospitalized during a 9-year period. A total of 46 sociodemographic and clinical characteristics were examined as potential predictors of 30-d readmission, expanding the existing body of literature (7,8,10,11,26,32) In addition, DERRI™ performance was similar between the training and validation samples. Lastly, we are unaware of any previously published model specifically designed for use prior to discharge that predicts 30-d readmission risk for patients with diabetes.
A key unanswered question is whether use of the DERRI™ to identify high-risk patients would reduce readmission risk in the context of an interventional program. Several intervention trials in various populations of medical patients have shown statistically significant relative risk reductions in 30-d readmissions (13,33-35) Of the successful studies, all but one tested multi-component discharge bundles, suggesting that bundled interventions may yield an additive benefit beyond that seen with a single intervention. Common components of these interventions were patient-centered discharge education, peridischarge coordination of care, and postdischarge support. Additional research is needed to develop and test such interventions in patients with diabetes. Targeting interventions to patients at high risk may optimize hospital-centered cost:benefit ratios comparing extended inpatient costs with penalties for early readmissions. It is possible that this tool could be used to automatically calculate risk by embedding it into the electronic medical record. One might envision that a high-risk patient could be flagged for additional services and support intended to reduce readmission risk. A DERRI™ calculator is available online at https://redcap.templehealth.org/redcap/surveys/?s=3XCPCAMKWE.
CONCLUSION
In summary, using a cohort of hospitalized patients with diabetes, we developed the DERRI™ model to predict all-cause 30-d readmission with acceptable predictive power. Because all the predictors in the DERRI™ are easily obtained on admission, this model could be used to identify patients at higher risk of 30-d readmission early in their hospitalization. The identification of high-risk patients may enable interventions to be targeted to those at greatest risk, potentially leading to better outcomes and lower costs by reducing hospital readmission rates.
ACKNOWLEDGMENT
The authors thank Kevin Jon Williams and Elias Siraj at the Lewis Katz School of Medicine at Temple University Section of Endocrinology, Diabetes, and Metabolism for their support of this work.
D.R. was supported by a Temple University Department of Medicine Junior Faculty Research Award and the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health (K23DK102963). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the study design, data, writing the report, or submission for publication.
Dr. Rubin receives research support from Boehringer Ingelheim, Astra Zeneca and Merck.
Abbreviations
- DERRI™
Diabetes Early Readmission Risk Indicator
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- GEE
generalized estimating equations
- ROC
receiver operating characteristic
Footnotes
DISCLOSURE
The other authors have no multiplicity of interest to disclose.
A prior iteration of this work was presented in abstract form at the American Diabetes Association 74th Scientific Sessions June 13-17, 2014 in San Francisco, California.
REFERENCES
- 1.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 2.Stone J, Hoffman G. Medicare Hospital Readmissions: Issues, Policy Options and PPACA. Congressional Research Service, Penny Hill Press; 2010. [Google Scholar]
- 3.Epstein AM. Revisiting readmissions--changing the incentives for shared accountability. N Engl J Med. 2009;360:1457–1459. doi: 10.1056/NEJMe0901006. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care. 2013 [Google Scholar]
- 5.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. U.S. Department of Health and Human Services; Atlanta, GA: 2014. 2014. Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 6.HCUP Nationwide Inpatient Sample (NIS) [Accessed February 1, 2016];Agency for Healthcare Research and Quality (AHRQ) 2015 2013. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp.
- 7.Robbins JM, Webb DA. Diagnosing diabetes and preventing rehospitalizations: the urban diabetes study. Med Care. 2006;44:292–296. doi: 10.1097/01.mlr.0000199639.20342.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett KJ, Probst JC, Vyavaharkar M, Glover SH. Lower rehospitalization rates among rural Medicare beneficiaries with diabetes. J Rural Health. 2012;28:227–234. doi: 10.1111/j.1748-0361.2011.00399.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Ma Q, Chen H, Yermilov I. New bundled world: quality of care and readmission in diabetes patients. J Diabetes Sci Technol. 2012;6:563–571. doi: 10.1177/193229681200600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eby E, Hardwick C, Yu M, et al. Predictors of 30 day hospital readmission in patients with type 2 diabetes: a retrospective, case–control, database study. Curr Med Res Opin. 2015;31:107–114. doi: 10.1185/03007995.2014.981632. [DOI] [PubMed] [Google Scholar]
- 11.Healy SJ, Black D, Harris C, Lorenz A, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care. 2013;36:2960–2967. doi: 10.2337/dc13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin DJ, Handorf E, McDonnell M. Predicting Early Readmission Risk among Hospitalized Patients with Diabetes (7796); Program of ENDO 2013: The Endocrine Society’s 95th Annual Meeting; San Francisco, CA. June 15-18, 2013; Abstract. [Google Scholar]
- 13.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 14.Draznin B, Gilden J, Golden SH, et al. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care. 2013;36:1807–1814. doi: 10.2337/dc12-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334:1441–1447. doi: 10.1056/NEJM199605303342206. [DOI] [PubMed] [Google Scholar]
- 16.Cook CB, Naylor DB, Hentz JG, et al. Disparities in diabetes-related hospitalizations: relationship of age, sex, and race/ethnicity with hospital discharges, lengths of stay, and direct inpatient charges. Ethn Dis. 2006;16:126–131. [PubMed] [Google Scholar]
- 17.Cramer S, Chapa G, Kotsos T, Jenich H. Assessing multiple hospitalizations for health-plan-managed Medicaid diabetic members. J Healthc Qual. 2010;32:7–14. doi: 10.1111/j.1945-1474.2010.00089.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Ross JS, Melkus GD, Zhao Z, Boockvar K. Scheduled and unscheduled hospital readmissions among patients with diabetes. Am J Manag Care. 2010;16:760–767. [PMC free article] [PubMed] [Google Scholar]
- 19.Menzin J, Korn JR, Cohen J, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm. 2010;16:264–275. doi: 10.18553/jmcp.2010.16.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:1432–1435. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi A, Peelen LM, Corpeleijn E, et al. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ. 2012;345:e5900. doi: 10.1136/bmj.e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25:3474–3486. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 24.Royston P, Moons KGM, Altman DG, Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 25.Jiang HJ, Andrews R, Stryer D, Friedman B. Racial/ethnic disparities in potentially preventable readmissions: the case of diabetes. Am J Public Health. 2005;95:1561–1567. doi: 10.2105/AJPH.2004.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rico F, Liu Y, Martinez DA, Huang S, Zayas-Castro JL, Fabri PJ. Preventable Readmission Risk Factors for Patients With Chronic Conditions. J Healthc Qual. 2015:1–16. doi: 10.1097/01.JHQ.0000462674.09641.72. [DOI] [PubMed] [Google Scholar]
- 27.Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20:870–875. doi: 10.4158/EP14006.OR. [DOI] [PubMed] [Google Scholar]
- 28.Chen HF, Popoola T, Radhakrishnan K, Suzuki S, Homan S. Improving diabetic patient transition to home healthcare: leading risk factors for 30-day readmission. Am J Manag Care. 2015;21:440–450. [PubMed] [Google Scholar]
- 29.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American College of Cardiology/American Heart Association [Accessed June 29, 2016];CV Risk Calculator. 2013 Available at: https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
- 31.Rubin DJ, Donnell-Jackson K, Jhingan R, Golden SH, Paranjape A. Early readmission among patients with diabetes: a qualitative assessment of contributing factors. J Diabetes Complications. 2014;28:869–873. doi: 10.1016/j.jdiacomp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht JS, Hirshon JM, Goldberg R, et al. Serious Mental Illness and Acute Hospital Readmission in Diabetic Patients. Am J Med Qual. 2012;27:503–508. doi: 10.1177/1062860612436576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4:211–218. doi: 10.1002/jhm.427. [DOI] [PubMed] [Google Scholar]
- 34.Coleman EA, Parry C, Chalmers S, Min S. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 35.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]