Abstract
(1) Background and Objectives
Patients with metastatic RCC can undergo metastasectomy to improve survival time. Our goal was to provide and compare characteristics and oncological outcomes of RCC patients who underwent complete metastasectomy at a single organ site.
(2) Methods
138 RCC patients were identified as undergoing complete metastasectomy at a single organ site including adrenal, lung, liver, pancreas, or thyroid. Competing risk regression analysis was used to assess RFS and CSS adjusting for several covariates.
(3) Results
In this highly selected cohort, RFS and CSS was 27% and 84% at five years following metastasectomy, respectively. Univariate analysis revealed that removal of multiple tumors, younger age, and a shorter interval between nephrectomy and metastasis was associated with worse RFS. Larger tumors and sarcomatoid histology at nephrectomy was associated with worse CSS. We found no evidence that metastases at the time of RCC diagnosis influenced recurrence or survival. Tumor size, number of metastases resected and time from nephrectomy to first recurrence was significantly different, but recurrence rates were not found to be significantly different, when compared across all organ sites.
(4) Conclusions
These findings inform clinical and surgical management of select RCC patients with isolated metastasis to one of several organ sites.
Keywords: metastasectomy for renal cell carcinoma, adrenal, liver, lung, pancreas, thyroid
Introduction
Metastatic RCC (mRCC) has a median survival time of 6 to 12 months and a 5-year survival rate of less than 20%.[1,2] About 25% of patients with RCC will present with metastatic disease and another 25% will develop metastatic disease following nephrectomy. Surgical resection of metastatic sites or metastasectomy has been associated in retrospective comparative studies with prolonged survival time. Five-year survival rates of at least 30% to 45% have been reported for patients receiving metastasectomy.[3–5] This increase in survival is seen even when mRCC patients are stratified using a prognostic scoring system and metastasectomy patients are compared with their counterparts within the same risk category.[6] The European Association of Urology recently recommended in their guidelines that metastasectomy should be considered for most metastatic sites with the exception of brain and possibly bone.[7]
Further prognostic factors have been reported that significantly influence survival of patients who undergo metastasectomy. In one of the original studies to investigate the significance of complete surgical resection of metastatic sites, complete, incomplete, and no surgical resection 5-year survival rates of 44%, 14%, and 11%, were reported, respectively.[4] Numerous retrospective studies presented in a recent systemic review had similar survival rates.[8] Complete resection has further been shown to improve survival if analyzed for liver, lung, or pancreas resections alone compared with incomplete or no resection at the specific metastatic site.[9–18]
Survival and recurrence data for individual organ sites has been presented in previous studies with the exception of adrenal. Previously reported 5-year survival rates following metastasectomy include 38–62% (liver),[10–12] 33–44% (lung),[14–17] 45–88% (pancreas),[13,18] and 51% (thyroid).[19,20] Although not necessarily compared with their non-resected counterparts, these survival rates are superior to overall mRCC five-year survival rates. In addition to this associated survival benefit, more studies describing individual organ sites of metastasectomy are important because RCC pathology represents a large percentage of all metastatic resections and represents the largest percentage at the adrenal, pancreas and thyroid in multiple studies.[13,21–25] To this end, we selected for patients from a single institution who underwent complete metastasectomy at a single organ site including adrenal, liver, lung, pancreas, and thyroid. The goal was to provide and compare patient and disease characteristics, as well as survival data, for the entire cohort and across these multiple organ sites to inform patient care.
Materials and Methods
After obtaining institutional review board approval, we identified patients who had previously undergone nephrectomy for RCC, had an RCC recurrence, and subsequently underwent metastasectomy at Memorial Sloan Kettering Cancer Center. The year of initial nephrectomy ranged from 1976 to 2012, and the year of metastasectomy ranged from 1990 to 2013. From this cohort we selected for patients who underwent complete metastasectomy at an isolated organ site. Complete was defined as no evidence of disease following metastasectomy. Isolated was defined as metastatic disease confined to one organ site. Patients who underwent previous metastatic resections, had widespread metastatic disease at time of metastasectomy, or had residual disease following resection were excluded. We further defined patients as having synchronous disease as those patients who had distant metastases at nephrectomy or within 6 months after nephrectomy or metachronous disease (all others). Recurrence was considered to be any new metastatic disease after nephrectomy or metastasectomy, determined by imaging or biopsy. Several patients underwent metastasectomy shortly before nephrectomy, and for these patients we considered time from nephrectomy to recurrence and metastasectomy as 0 days. With these definitions, all analyzed patients had a complete metastasectomy of an isolated tumor from the adrenal gland, lung, liver, pancreas, or thyroid.
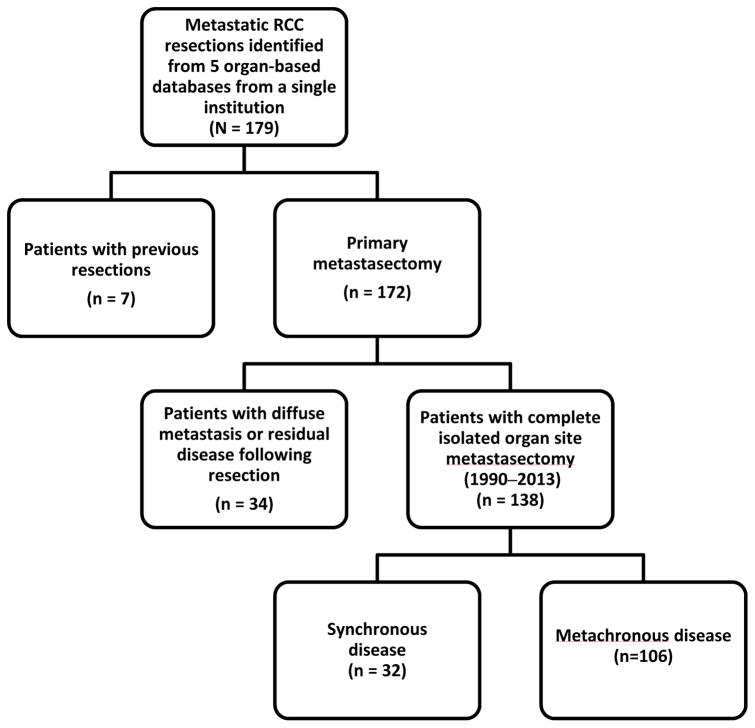
Using five organ-based databases from a single institution, we identified a total of 179 patients who underwent metastasectomy for mRCC. Further selection of the final analyzed cohort is described in Figure I. Seven patients with previous metastatic resections were excluded, and 34 patients with widespread metastatic disease at time of metastasectomy or residual disease following resection were also excluded from the original cohort. After exclusions, 138 patients with complete metastasectomy at an isolated organ site were included in this study, with 32 patients having synchronous disease and 106 having metachronous disease.
Figure I.
Cohort selection schematic.
Our goal was to determine which patient and disease characteristics were associated with recurrence free survival (RFS) and cancer specific survival (CSS). We used competing risk regression, with death that is not caused by cancer as a competing risk for recurrence and death from disease, to examine the association between RFS and CSS and our covariates of interest: metastasis at time of RCC diagnosis, age at metastasectomy, time from nephrectomy to metastasis, time from metastasis to metastasectomy, number of tumors removed at metastasectomy (single or multiple), maximum tumor size at metastasectomy, sarcomatoid histology, Karnofsky Performance Score (KPS), and site of metastasectomy. Log rank tests were used to test for differences in RFS based on site of metastasis. Reported KPS scores were within 1 year of metastasectomy. The association between site of metastasectomy and CSS was not assessed because of a limited number of events. We also compared these covariates by site of metastasis to determine whether there were differences in disease characteristics or patient selection. A sensitivity analysis using univariate Cox models without adjustment for competing risks was also performed. Kaplan-Meier (KM) methods were used to estimate RFS and CSS for the entire cohort. All analyses were completed using Stata 13.0 (StataCorp, College Station, TX).
Results
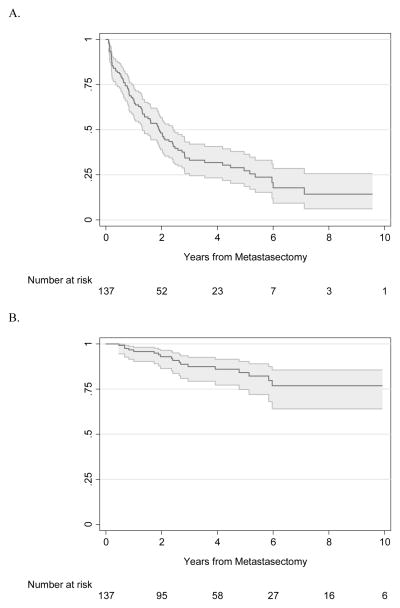
Characteristics of our patient cohort are reported in Table I. In this cohort of 138 patients, 89 patients experienced recurrence after metastasectomy, and 54 patients died from any cause, with 19 (14%) of these reported deaths caused by RCC (Table I). The median follow-up period was 3.0 years after metastasectomy (interquartile range [IQR] 1.6, 5.8) for survivors. Regarding systemic therapy, treatment at our institution was confirmed in 54% of the cohort. Performing temporal analysis on our cohort we found no evidence that having a nephrectomy before 2005 (N=43) or after 2005 (N=95) was associated with risk of RFS (p=0.11), OS (p>0.9), or CSS (p=0.5). RFS and CSS rates from metastasectomy were 48% (95% confidence interval [CI] 39%, 57%) and 93% (95% CI 86%, 96%) at 2 years, and 27% (95% CI 19%, 36%) and 84% (95% CI 75%, 90%) at 5 years, respectively. The Kaplan-Meier RFS and CSS curves for the entire cohort are shown in Figure II.
Table I.
Patient characteristics, n = 138. Data reported as median (interquartile range (IQR)) or frequency (percent (%)).
| Female sex, n (%) | 48 (35%) |
| Age at metastasectomy, y (IQR) | 64 (56, 70) |
| Any clear cell histology at nephrectomy, n (%) | 108 (78%) |
| Only clear cell histology at nephrectomy, n (%) | 97 (70%) |
| Sarcomatoid histology at nephrectomy, n (%) | 8 (5.8%) |
| Synchronous disease, n (%) | 30 (22%) |
| Site of metastasectomy, n (%) | |
| Adrenal | 27 (20%) |
| Liver | 12 (8.7%) |
| Lung | 78 (57%) |
| Pancreas | 15 (11%) |
| Thyroid | 6 (4.3%) |
| Multiple tumors removed at metastasectomy, n (%) | 51 (37%) |
| Karnofsky Performance Score (n = 89), n (%) | |
| ≤75 | 3 (3.3%) |
| 80 | 17 (19%) |
| 85 | 2 (2.2%) |
| 90 | 58 (65%) |
| 100 | 9 (10%) |
| Confirmed systemic therapy, n (%) | 74 (54%) |
Figure II.
A. Kaplan-Meier survival estimates with 95% CI for RFS after metastasectomy. B. Kaplan-Meier survival estimates with 95% CI for CSS after metastasectomy.
On univariate analysis, patients who had more than one tumor removed from a single organ site at the time of metastasectomy had a higher risk of recurrence (hazard ratio (HR) 1.95, 95% CI 1.26, 3.03, p=0.003), whereas older patients and those patients with a longer interval between nephrectomy and metastasis were at decreased risk of recurrence after metastasectomy (HR 0.74 per 10 years, 95% CI 0.59, 0.94, p=0.012, and HR 0.95 per 10 years, 95% CI 0.90, 0.99, p=0.023, respectively). Larger tumors at metastasectomy (HR 1.18 per 1 cm, 95% CI 1.07, 1.29, p=0.001) and the presence of sarcomatoid histology (HR 3.70, 95% CI 1.09, 12.62, p=0.037) were significantly associated with worse CSS (Table II). Although time from nephrectomy to metastasis was significantly associated with RFS, we found no evidence of an association with CSS. We also found no evidence that metastases at the time of RCC diagnosis or time from diagnosis of metastasis to metastasectomy was associated with either RFS or CSS. KPS was also not significantly associated with either oncologic outcome. However, our cohort is highly selected, composed of patients who were deemed healthy enough for metastasectomy. As such, there was little variation in KPS, with 75% of patients in this cohort having a KPS ≥90. A sensitivity analysis using univariate Cox models without competing risks led to similar results.
Table II.
Univariate competing risk regression models for recurrence-free survival and cancer-specific survival (HR = hazard ratio).
| RFS | CSS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Synchronous disease | 1.39 | 0.88, 2.20 | 0.2 | 1.34 | 0.47, 3.81 | 0.6 |
| Metastases at RCC diagnosis | 1.38 | 0.85, 2.26 | 0.2 | 0.82 | 0.23, 2.96 | 0.8 |
| Age at metastasectomy (per 10 years) | 0.74 | 0.59, 0.94 | 0.012 | 0.95 | 0.59, 1.52 | 0.8 |
| Time from nephrectomy to metastasis (per year) | 0.95 | 0.90, 0.99 | 0.023 | 0.99 | 0.91, 1.08 | 0.8 |
| Time from metastasis to metastasectomy (per year) | 1.00 | 0.97, 1.03 | >0.9 | 0.96 | 0.90, 1.03 | 0.3 |
| Multiple tumors removed at metastasectomy | 1.95 | 1.26, 3.03 | 0.003 | 0.93 | 0.38, 2.27 | 0.9 |
| Maximum tumor size at metastasectomy (cm) | 1.05 | 0.99, 1.11 | 0.080 | 1.18 | 1.07, 1.29 | 0.001 |
| Sarcomatoid histology | 1.81 | 0.74, 4.40 | 0.2 | 3.70 | 1.09, 12.62 | 0.037 |
| KPS (per 10 patients) | 0.98 | 0.94, 1.02 | 0.3 | 1.00 | 0.95, 1.05 | >0.9 |
| Metastasectomy site | ||||||
| Adrenal | Ref. | - | 0.14 | - | - | - |
| Liver | 1.10 | 0.48, 2.52 | - | - | - | |
| Lung | 1.55 | 0.93, 2.58 | - | - | - | |
| Pancreas | 0.68 | 0.32, 1.48 | - | - | - | |
| Thyroid | 0.91 | 0.31, 2.70 | - | - | - | |
We further compared clinicopathological variables across separate organ sites in Table III. The lung was the most common site of metastasectomy (n=78, 57%) followed by adrenal (n=27, 20%), liver (n=12, 9%), pancreas (n=15, 11%), and thyroid (n=6, 4%). We found a significant difference between sites regarding tumor size, number of metastases resected, and time from nephrectomy to first recurrence. Despite a difference in tumor and disease characteristics by site, there was not a significant difference in RFS between the sites. The 5-year RFS probabilities by site were 32%, 27%, 22%, and 43% for adrenal, liver, lung, and pancreas sites, respectively (p=0.14; Table III).
Table III.
Patient and disease characteristics by site of metastasectomy. Data reported as frequency (percent) or median (interquartile range). RFS is reported with 95% CI.
| Adrenal (n=27; 20%) | Liver (n=12; 8.7%) | Lung (n=78; 57%) | Pancreas (n=15; 11%) | Thyroid (n=6; 4.3%) | p-value | |
|---|---|---|---|---|---|---|
| Age at metastasectomy, years | 65 (57, 71) | 64 (58, 70) | 60 (54, 68) | 69 (64, 78) | 64 (59, 76) | 0.029 |
| Tumor size (cm) (n=136) | 4.1 (2.5, 6.2) | 5.6 (3.7, 8.5) | 1.5 (0.9, 2.4) | 2.8 (1.5, 4.5) | 2.2 (1.3, 2.5) | <0.0001 |
| Number of metastases resected | ||||||
| 1 | 25 (93%) | 9 (75%) | 38 (49%) | 10 (67%) | 5 (83%) | 0.001 |
| >1 | 2 (7.4%) | 3 (25%) | 40 (51%) | 5 (33%) | 1 (17%) | |
| Disease presentation, n (%) | ||||||
| Synchronous | 8 (30%) | 3 (25%) | 16 (21%) | 1 (6.7%) | 2 (33%) | 0.5 |
| Metachronous | 19 (70%) | 9 (75%) | 62 (79%) | 14 (93%) | 4 (67%) | |
| Time from nephrectomy to recurrence (years) | 5.1 (2.8, 7.6) | 7.8 (4.0, 11.0) | 2.4 (1.2, 6.7) | 9.0 (8.5, 21.3) | 4.7 (3.4, 5.0) | 0.045 |
| Time from recurrence to metastasectomy (months) | 2.5 (1.8, 13.3) | 2.5 (2.3, 5.7) | 3.6 (1.5, 7.5) | 1.8 (1.0, 6.1) | 1.4 (0.7, 3.7) | 0.3 |
| 5-year RFS rate | 32% (13%, 52%) | 27% (2%, 66%) | 22% (12%, 34%) | 43% (16%, 67%) | * | 0.14 |
All patients either recurred, died, or were lost to follow-up at 5 years.
Discussion
In this study, we observed a CSS rate of 93% at 2 years and 84% at 5 years for a carefully selected group of patients who underwent metastasectomy. The selection factors included isolated organ metastasis and complete metastasectomy. Regarding timing of metastatic disease, we found no evidence that metastases at the time of RCC diagnosis (synchronous disease) or time from nephrectomy to metastasis affects survival. A longer disease-free interval following nephrectomy has been previously shown to be a positive prognostic factor.[4,15] These previous studies, however, did not involve the same selection criteria used in this study. Our findings suggest that resection should be attempted at a single organ site if complete resection can be achieved regardless of the length of the disease-free interval.
In this cohort, having multiple tumors at an organ site was associated with worse RFS, while RFS was better for older patients and patients with a longer interval between nephrectomy and metastasis. Larger tumor size and sarcomatoid histology was associated with reduced CSS. Sarcomatoid histology is an extremely aggressive histologic variant of RCC with a median survival of only 4 to 12 months.[26] Previously reported predictors of survival following metastasectomy include a single site of first recurrence following nephrectomy, complete resection of first metastasis, a long disease-free interval following nephrectomy and a metachronous presentation with recurrence.[4] All patients in our cohort had a complete resection of metastasis at a single site, and we found no evidence that a longer disease-free interval from nephrectomy or metachronous presentation provided additional survival benefit in these patients. Although there was no evidence of a difference in RFS by site, clinicopathological features described here give insight into organ site-specific selection factors and provide further guidance in patient selection.
Our study had several limitations. Considerable selection bias in this study affects the ability to determine whether metastasectomy truly alters the natural history of this favorable group of patients and the heterogeneous biology of mRCC. Lymph node metastasis and resections were not analyzed in this study, as the focus was on solid organ metastasis and resection. There were a limited number of patients and cancer related deaths in certain organ resection groups. The lung patient group represented 57% of the combined cohort; thus, the results in this study are biased toward the outcomes for lung metastasectomy patients. These factors further influenced our ability to create an appropriate multivariable model or perform univariate analyses by site of metastasectomy.
Systemic therapy could not be reliably recorded because many patients underwent treatment at other institutions. When performing temporal analysis on our cohort we found no evidence of a difference in RFS or CSS between patients who were treated before and after 2005, when the use of systemic therapy became more common. Since 2005 the FDA has approved several agents that target angiogenesis or the mTOR pathway. This cutoff year has been used in two studies utilizing Survival, Epidemiology, and End Results (SEER) data that compared survival of patients prior to and after 2005.[27,28] It must be noted that many of these agents have only improved OS marginally, rarely cause complete response and are not curative.[29] Although encouraging, the moderate increases in survival following the introduction of targeted therapies further stress the importance of metastasectomy as part of the treatment algorithm for mRCC.
Conclusions
This study presents a select group of mRCC patients with prolonged survival who underwent complete metastasectomy. We describe disease characteristics and selection factors associated with recurrence and survival and further differentiate these characteristics across several organ sites. The information in this study informs clinicians in selecting patients who are appropriate for metastasectomy and in guiding further patient care following resection.
Synopsis.
Carefully selected renal cell carcinoma patients with isolated metastases undergoing metastasectomy can experience prolonged survival time. This study describes a competing risk analysis in patients undergoing complete metastasectomy from 5 organ sites.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30CA008748.
Abbreviations list
- RCC
renal cell carcinoma
- mRCC
metastatic renal cell carcinoma
- RFS
recurrence free survival
- CSS
cancer specific survival
- KPS
Karnofsky performance score
- KM
Kaplan-Meier
- IQR
Interquartile range
- CI
confidence interval
- HR
Hazard Ratio
Footnotes
No disclosures
References
- 1.Flanigan RC, Campbell SC, Clark JI, Picken MM. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385–390. doi: 10.1007/s11864-003-0039-2. [DOI] [PubMed] [Google Scholar]
- 2.Swanson DA. Surgery for metastases of renal cell carcinoma. Scand J Surg SJS Off Organ Finn Surg Soc Scand Surg Soc. 2004;93:150–155. doi: 10.1177/145749690409300211. [DOI] [PubMed] [Google Scholar]
- 3.Eggener SE, Yossepowitch O, Kundu S, Motzer RJ, Russo P. Risk score and metastasectomy independently impact prognosis of patients with recurrent renal cell carcinoma. J Urol. 2008;180:873–878. doi: 10.1016/j.juro.2008.05.006. discussion 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 5.Kierney PC, van Heerden JA, Segura JW, Weaver AL. Surgeon’s role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol. 1994;1:345–352. doi: 10.1007/BF02303572. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TBL, Canfield SE, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549–561. doi: 10.1016/S1470-2045(14)70235-9. [DOI] [PubMed] [Google Scholar]
- 9.Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–2882. doi: 10.1002/cncr.25836. [DOI] [PubMed] [Google Scholar]
- 10.Staehler MD, Kruse J, Haseke N, Stadler T, Roosen A, Karl A, et al. Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J Urol. 2010;28:543–547. doi: 10.1007/s00345-010-0560-4. [DOI] [PubMed] [Google Scholar]
- 11.Ruys AT, Tanis PJ, Iris ND, van Duijvendijk P, Verhoef C, Porte RJ, et al. Surgical Treatment of Renal Cell Cancer Liver Metastases: A Population-Based Study. Ann Surg Oncol. 2011;18:1932–1938. doi: 10.1245/s10434-010-1526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thelen A, Jonas S, Benckert C, Lopez-Hänninen E, Rudolph B, Neumann U, et al. Liver resection for metastases from renal cell carcinoma. World J Surg. 2007;31:802–807. doi: 10.1007/s00268-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 13.Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, et al. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008;15:3199–3206. doi: 10.1245/s10434-008-0140-7. [DOI] [PubMed] [Google Scholar]
- 14.Pfannschmidt J, Hoffmann H, Muley T, Krysa S, Trainer C, Dienemann H. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg. 2002;74:1653–1657. doi: 10.1016/s0003-4975(02)03803-1. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann HS, Neef H, Krohe K, Andreev P, Silber RE. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol. 2005;48:77–81. doi: 10.1016/j.eururo.2005.03.004. discussion 81–82. [DOI] [PubMed] [Google Scholar]
- 16.Piltz S, Meimarakis G, Wichmann MW, Hatz R, Schildberg FW, Fuerst H. Long-term results after pulmonary resection of renal cell carcinoma metastases. Ann Thorac Surg. 2002;73:1082–1087. doi: 10.1016/s0003-4975(01)03602-5. [DOI] [PubMed] [Google Scholar]
- 17.Fourquier P, Regnard JF, Rea S, Levi JF, Levasseur P. Lung metastases of renal cell carcinoma: results of surgical resection. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 1997;11:17–21. doi: 10.1016/s1010-7940(96)01013-5. [DOI] [PubMed] [Google Scholar]
- 18.Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic Metastasis From Renal Cell Carcinoma: Which Patients Benefit From Surgical Resection? Ann Surg Oncol. 2008;15:1161–1168. doi: 10.1245/s10434-007-9782-0. [DOI] [PubMed] [Google Scholar]
- 19.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95:1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 20.Iesalnieks I, Winter H, Bareck E, Sotiropoulos GC, Goretzki PE, Klinkhammer-Schalke M, et al. Thyroid metastases of renal cell carcinoma: clinical course in 45 patients undergoing surgery. Assessment of factors affecting patients’ survival. Thyroid Off J Am Thyroid Assoc. 2008;18:615–624. doi: 10.1089/thy.2007.0343. [DOI] [PubMed] [Google Scholar]
- 21.Muth A, Persson F, Jansson S, Johanson V, Ahlman H, Wängberg B. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol EJSO. 2010;36:699–704. doi: 10.1016/j.ejso.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287–293. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]
- 23.Untch BR, Allen PJ. Pancreatic metastasectomy: the Memorial Sloan-Kettering experience and a review of the literature. J Surg Oncol. 2014;109:28–30. doi: 10.1002/jso.23460. [DOI] [PubMed] [Google Scholar]
- 24.Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–578. doi: 10.1002/(sici)1097-0142(19970201)79:3<574::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2004;30:583–588. doi: 10.1016/j.ejso.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid Renal Cell Carcinoma: A Comprehensive Review of the Biology and Current Treatment Strategies. The Oncologist. 2012;17:46–54. doi: 10.1634/theoncologist.2011-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal SK, Nelson RA, Vogelzang N. Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PloS One. 2013;8:e63341. doi: 10.1371/journal.pone.0063341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conti SL, Thomas IC, Hagedorn JC, Chung BI, Chertow GM, Wagner TH, et al. Utilization of cytoreductive nephrectomy and patient survival in the targeted therapy era. Int J Cancer J Int Cancer. 2014;134:2245–2252. doi: 10.1002/ijc.28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppin C, Le L, Porzsolt F, Wilt T. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev. 2008:CD006017. doi: 10.1002/14651858.CD006017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]