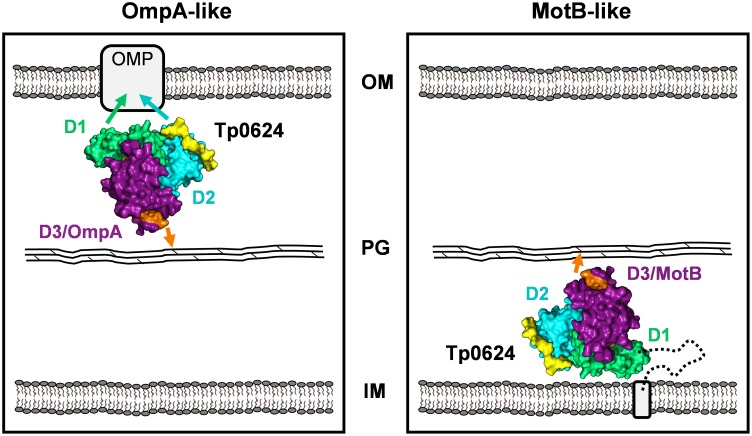
Fig 5. Model of the potential roles of Tp0624 in T. pallidum peptidoglycan binding and cell envelope structure stabilization.
(Left) OmpA-like model: Tp0624 is exported across the inner membrane (IM) followed by cleavage and removal of the signal peptide. Tp0624 binds peptidoglycan (PG) via non-covalent interactions (orange arrow) involving the OmpA-like D3 while D1 and/or D2 interact with outer membrane-localized proteins (OMP). Residues from the treponemal conserved TAD(x)6E/QxxLSxxRA motif (putative non-canonical peptidoglycan binding motif) and surrounding pocket located within D3 are shown in orange (Right) MotB-like model: Tp0624 is translocated across the inner membrane, however the signal peptide is not cleaved and the protein remains anchored to the inner membrane, but with the functional head group directed into the periplasm. Tp0624 binds peptidoglycan via its OmpA-like domain, forming a structural bridge with the inner membrane. In this subcellular location, Tp0624 could function in cell envelope and/or flagellar stabilization.