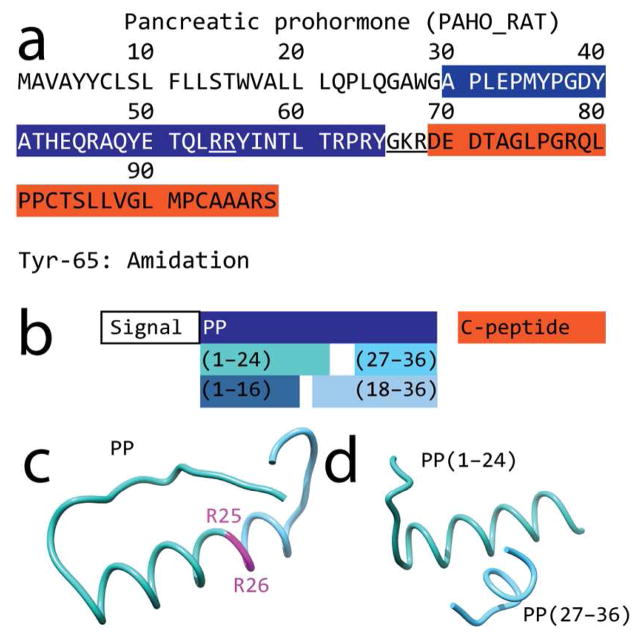
Figure 3.
Sequence and predicted structure of peptides originating from the rat pancreatic prohormone. (a) Sequence of rat pancreatic prohormone (UNIPROT ID: P06303, PAHO_RAT); the dibasic sites are underlined. (b) Observed peptide products resulting from processing of the pancreatic prohormone (filled color boxes). (c) Ab initio-calculated structure of PP; the dibasic site is outlined in magenta. (d) Molecular modeling shows that the N- and C-terminal products (sea green and sky blue, respectively) resulting from cleavage at the dibasic site retain the α-helix folding of the parent peptide.