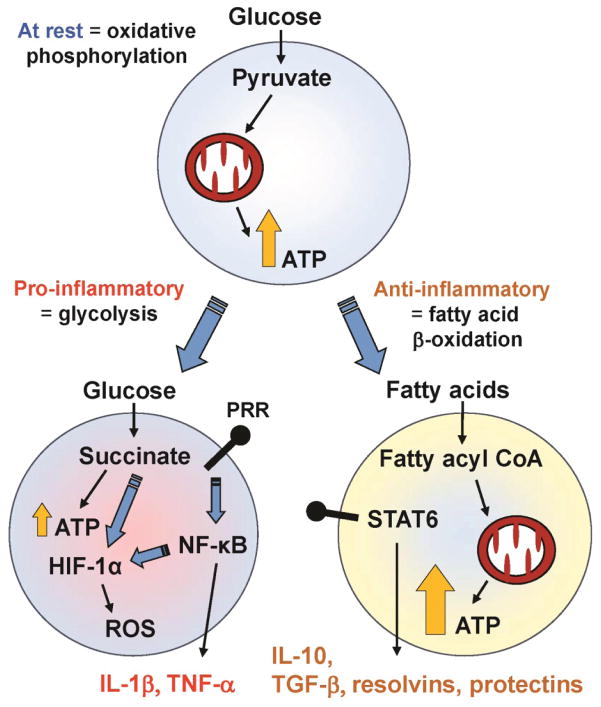
Figure 2. Metabolic Adaptation Occurring during Immune Activation.
Resting immune cells, such as monocytes or T cells primarily produce energy (ATP) from breaking down glucose into pyruvate, and through mitochondrial oxidative phosphorylation. The activation of pattern recognition receptors (PRRs) by microbes leads to the activation of the master transcription regulator NF-κB, which in turn results in inflammatory cytokine gene expression and production (e.g. IL-1β, TNF-α). Concurrently, cells also undergo a metabolic shift to aerobic glycolysis (a phenomenon also known as the Warburg effect), where energy production occurring through glycolysis breaks down glucose into succinate. Although less energy is produced for each glucose molecule, this pathway does provide large quantities of anabolic substrates necessary to feed into the high gene transcriptional activity occurring during immune activation. Succinate and NF-κB also stabilize the transcription factor HIF-1α, resulting in further upregulation of inflammatory cytokine gene expression. The shift away from oxidative phosphorylation also allows these cells to produce reactive oxygen species (ROS) more efficiently. By contrast, anti-inflammatory signaling (via STAT6) causes an increase in fatty acid uptake, mitochondrial biogenesis, and increased mitochondrial function. This metabolic shift towards fatty acid oxidation leads to upregulation of anti-inflammatory cytokines (e.g., IL-10, TGF-β) and pro-resolving lipid mediators such as resolvins and protectins.