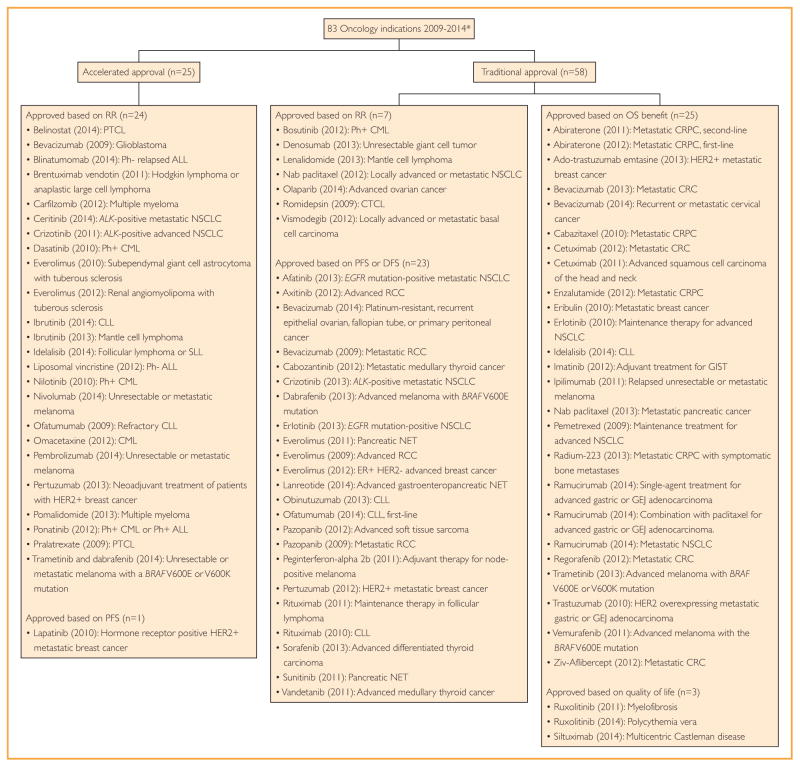
FIGURE 1.
All indications receiving Food and Drug Administration marketing authorization for oncology drugs between 2009 and 2014. Approvals are grouped based on traditional or accelerated authorization and the efficacy end point met to garner approval. *Drugs approved based on bioequivalence (mercaptopurine (2014): ALL, asparaginase Erwinia chrysanthemi (2011): ALL) were removed from the analysis. ALL = acute lymphoblastic leukemia; CLL = chronic lymphocytic lymphoma; CML = chronic myeloid leukemia; CRC = colorectal carcinoma; CRPC = castration-resistant prostate cancer; CTCL = cutaneous T-cell lymphoma; DFS = disease-free survival; GEJ = gastroesophageal junction; GIST = gastrointestinal stromal tumor; HER2 = human epidermal growth factor receptor 2; NET = neuro-endrocine tumor; NSCLC = non-small cell lung cancer; OS = overall survival; PFS = progression-free survival; Ph = Philadelphia chromosome; PTCL = peripheral T-cell lymphoma; RCC = renal cell cancer; RR = response rate; SLL = small lymphocytic lymphoma.