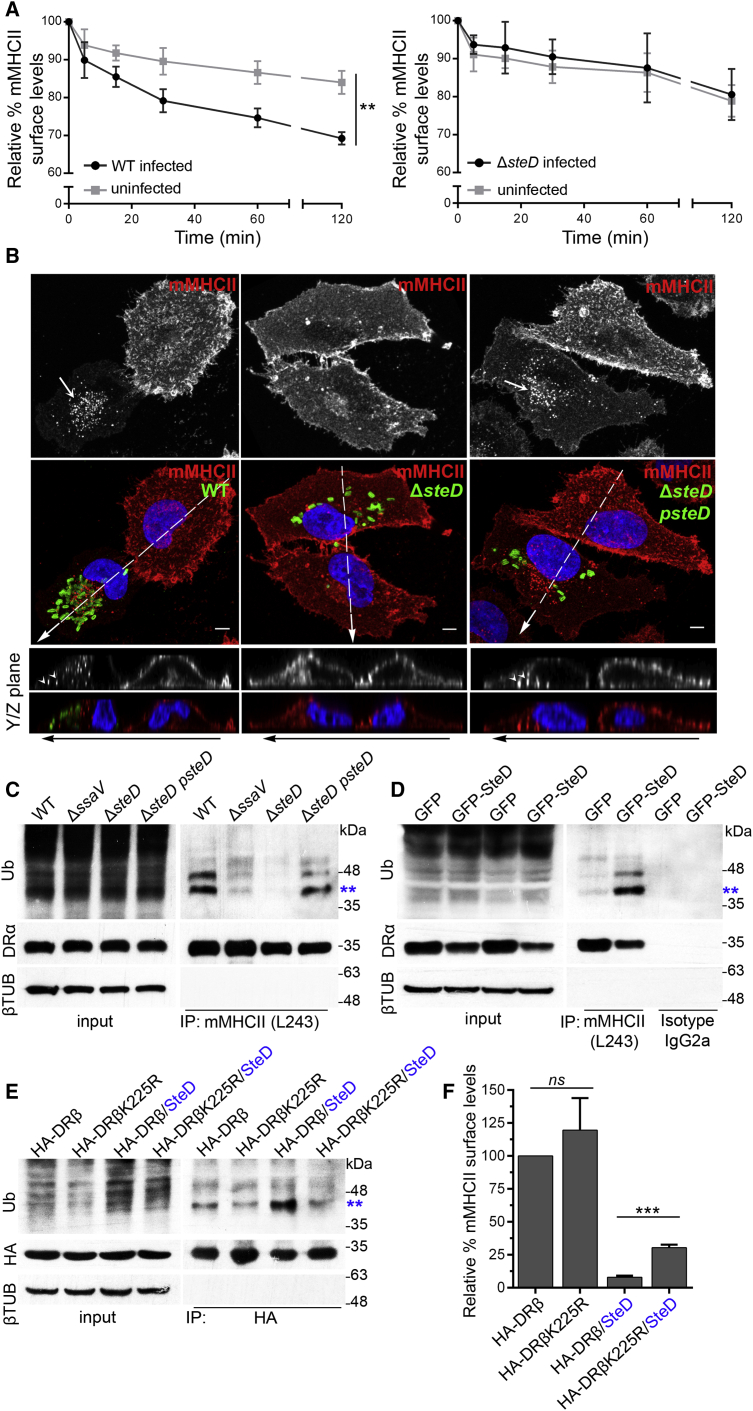
Figure 3.
SteD Depletes Surface Levels of mMHCII and Increases Its Ubiquitination
(A) Flow cytometry analysis showing surface mMHCII in Mel Juso cells infected with WT-GFP or ΔsteD-GFP strains compared to uninfected cells. The cells were infected for 16 hr and then labeled with mAb L243 on ice for 30 min to block internalization. The cells were exposed to 37°C to enable resumption of internalization. The surface levels of mMHCII at various time points were normalized to those at the time of transfer to 37°C (0 min), which represents 100%.
(B) Cells at 16 hr post invasion were labeled with mAb L243 on ice and incubated for another 4 hr in complete medium at 37°C. The cells were fixed and processed for immunofluorescence microscopy with DAPI nuclear stain (blue), anti-Salmonella CSA-1 (green), and anti-mouse secondary antibody against L243 (red). The images are maximum intensity Z projections showing the difference in mMHCII surface levels between cells infected with WT or ΔsteD psteD and its intracellular accumulation (arrows). The scale bar represents 5 μm. The regions indicated by white dashed lines of Z projections are shown below in the YZ plane. The internalized mMHCII in cells infected with WT or ΔsteD psteD strains are indicated by arrowheads.
(C) Mel Juso cells were infected at an MOI of 300:1 and at 16 hr post invasion lysed for immunoprecipitation with mAb L243 followed by western blot analysis with anti-ubiquitin (P4D1-HRP), anti-DRα, and anti-β tubulin antibodies.
(D) Stable Mel Juso cell lines expressing GFP or GFP-SteD were lysed and mAb L243 or IgG2a isotype control was used for immunoprecipitation. The immunoprecipitates were analyzed by western blot with the same antibodies as in (C).
(E) HA-tagged DRβ constructs (either wild-type [HA-DRβ] or with an arginine substitution of lysine 225 on DRβ cytoplasmic tail [HA-DRβ K225R]) were used to transduce Mel Juso cells or stable cells expressing GFP-SteD. The cells were lysed and immunoprecipitated with anti-HA antibody coupled to agarose beads followed by western blot analysis with anti-HA, anti-ubiquitin (P4D1-HRP), and anti-β tubulin antibodies. The blot shown is representative of three separate experiments in which band intensities, normalized to corresponding HA bands, are 1.00 ± 0.02 (DRβ), 0.59 ± 0.06 (DRβK225), 1.42 ± 0.44 (DRβ/SteD), and 0.50 ± 0.13 (DRβK225/SteD) (Student’s t test p < 0.03, comparison between DRβ/SteD and DRβK225/SteD). Double asterisks in (C)–(E) denote di-ubiquitinated DRβ.
(F) Surface levels of mMHCII on stable cells described in (E), measured by mAb L243 labeling and flow cytometry. The data shown are surface levels of mMHCII relative to that in cells expressing HA-DRβ. ∗∗∗p < 0.001, ∗∗p < 0.01, and not significant, ns (Student’s t test). The data shown are surface levels of mMHCII relative to that in cells expressing HA-DRβ.