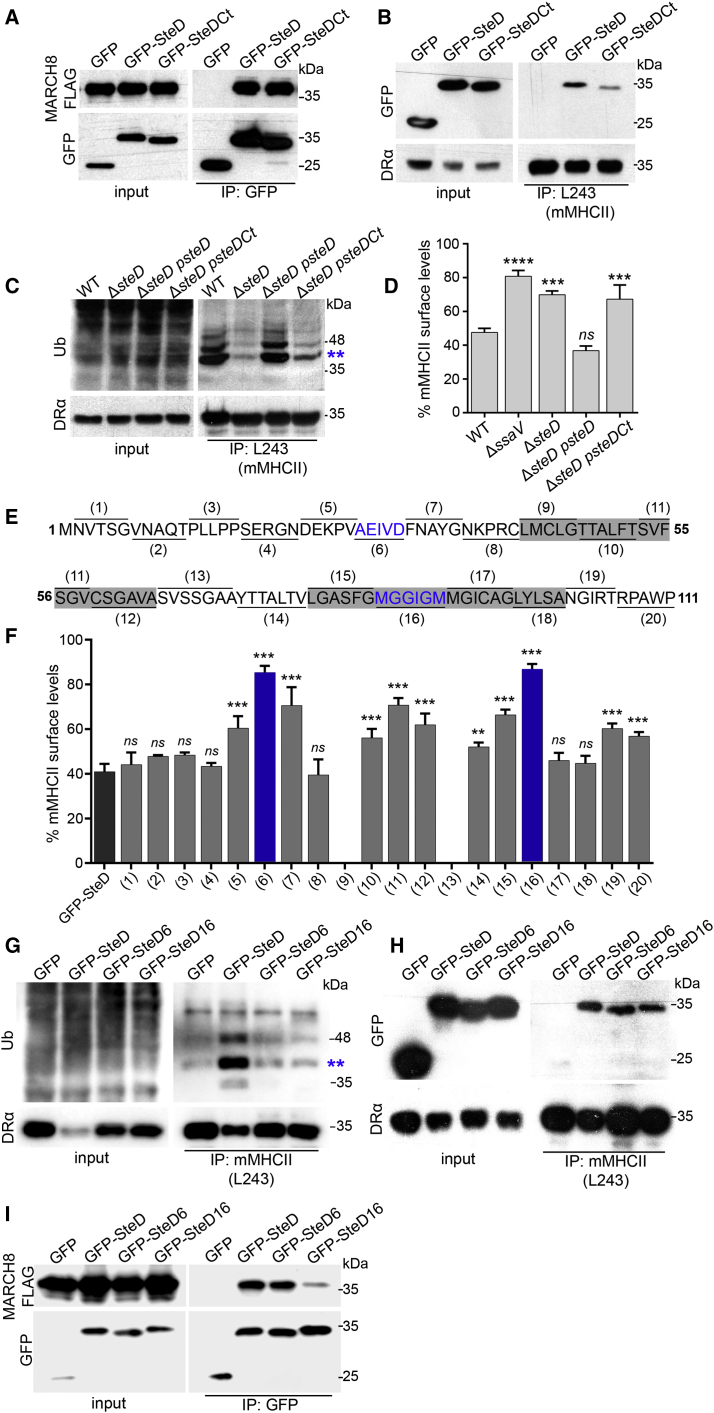
Figure 5.
Mutational Analysis of SteD
(A) HEK293T cells transfected with vectors expressing MARCH8-FLAG and GFP, GFP-SteD, or GFP-SteDCt were used for immunoprecipitation with GFP-Trap beads and analyzed by western blot with anti-GFP and anti-FLAG antibodies.
(B) Stable Mel Juso cell lines expressing GFP, GFP-SteD, and GFP-SteDCt were used for immunoprecipitation with mAb L243 (for mMHCII). The immunoprecipitates were analyzed by western blot with anti-GFP and anti-DRα antibodies. The blot shown is representative of three separate experiments in which the mean intensity of GFP-SteDCt compared to GFP-SteD after immunoprecipitation was 0.21 ± 0.14 (p < 0.02).
(C) Mel Juso cells were infected at an MOI of 300:1 and at 16 hr post invasion lysed for immunoprecipitation with mAb L243 followed by western blot analysis with anti-ubiquitin (P4D1-HRP) and anti-DRα antibodies.
(D) Mel Juso cells were transfected with vectors encoding GFP, GFP-SteD, and GFP-SteDCt, and mMHCII surface levels were analyzed by flow cytometry. The data represent surface levels of mMHCII in transfected cells as a percentage of those in untransfected cells from the same sample and are the means ± SD from three independent experiments.
(E) Schematic representation of SteD amino acids substituted with alanine. The transmembrane regions are shaded in gray.
(F) Mel Juso cells were transfected with vectors encoding mutated versions of SteD fused to GFP, and mMHCII surface levels were analyzed by flow cytometry. The data were analyzed as for (D) above.
(G) Stable Mel Juso cell lines expressing GFP, GFP-SteD, GFP-SteD6, or GFP-SteD16 were lysed and mAb L243 was used for immunoprecipitation and analysis as in (C).
(H) The stable cells used in (G) were used for immunoprecipitation with mAb L243 (for mMHCII) and analyzed as in (B).
(I) HEK293T cells transfected with vectors expressing MARCH8-FLAG and GFP, GFP-SteD, GFP-SteD6, or GFP-SteD16 were used for immunoprecipitation with GFP-Trap beads and analyzed as in (A). The blot shown is representative of three separate experiments in which the mean intensity of GFP-SteD16 compared to GFP-SteD after immunoprecipitation was 0.12 ± 0.07 (p < 0.02). The data in (D) and (F) were compared to GFP-SteD by one-way ANOVA followed by Dunnett’s multiple comparison test. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, and not significant, ns.