Figure 5.
MTRAP Is Dispensable for P. falciparum Asexual Stages
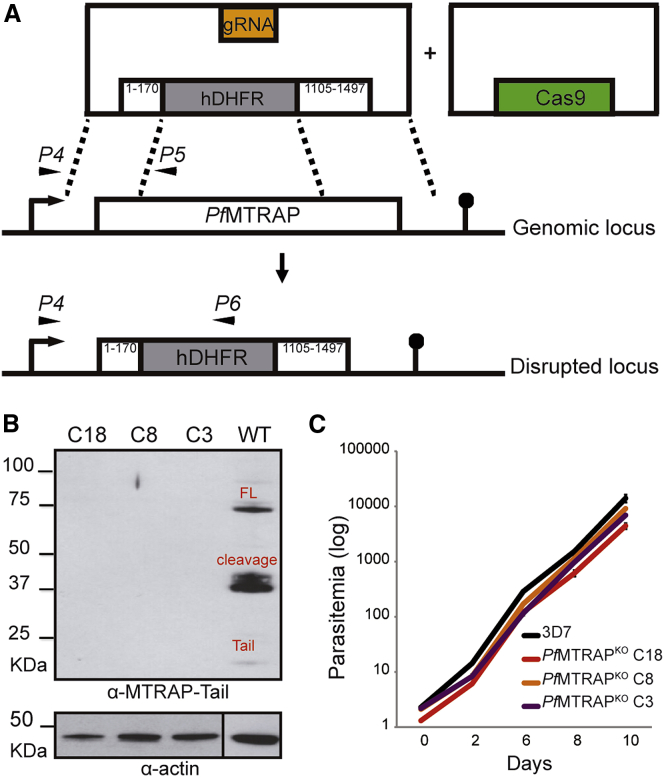
(A) Illustration of the strategy used to target P. falciparum mtrap for disruption. Two plasmids were transfected in the P. falciparum 3D7 strain, one plasmid carrying a guide DNA sequence (GAATGGTCAGAATGTAAAGA) and a hDHFR cassette flanked by two homology regions with the 5′and 3′ sequences of the mtrap coding sequence as indicated in the figure, and the second plasmid bearing a cassette for Cas9 expression. Double homologous recombination replaces 935 base pairs of the mtrap coding sequence by the hDHFR cassette, creating a disrupted locus. Primers used for PCR specific detection of the genomic or the disrupted loci are shown. See also Figure S2.
(B) Western blot analysis of the PfMTRAPKO clones C3, C8, and C18 with a specific antibody recognizing the MTRAP C-terminal region (α-MTRAP-Tail). Wild-type P. falciparum 3D7 strain (WT) was used as control. The α-MTRAP-Tail recognizes three specific bands in WT parasites, FL as the full-length protein, cleavage as a processed fragment, and Tail as the C-terminal region after processing. No bands are recognized in the three PfMTRAPKO clones. Actin (α-actin) was used as loading controls.
(C) Growth curves assessed every 48 hr by flow cytometry in blood cultures of P. falciparum wild-type (3D7, black line) or the three PfMTRAPKO clones (colored lines). The experiment was performed in triplicate and the data are presented as mean ± SD.