FIGURE 1.
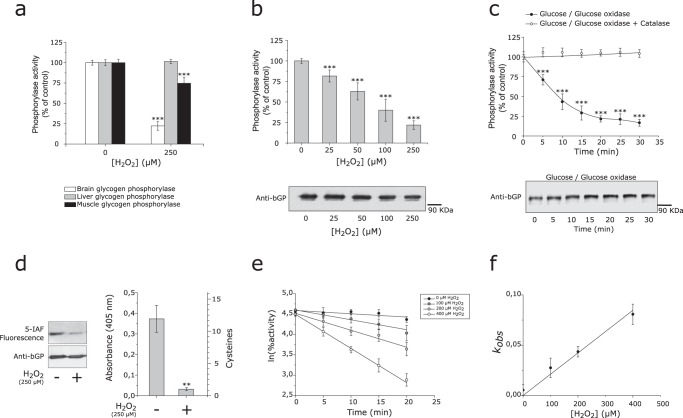
Human bGP is inhibited by H2O2 through modification of cysteine residues. a, purified recombinant human bGP and lGP (white and gray bars) or purified muscle glycogen phosphorylase (mGP) (rabbit) (black bars) were incubated with or without 250 μm H2O2 for 30 min at 37 °C, prior to residual activity measurement. mGP and bGP activity was measured using AMP as an activator, whereas lGP, which only responds to phosphorylation, was phosphorylated by phosphorylase kinase for activation prior to activity measurement. Results are expressed as the percentage of the control without H2O2. Data represent mean values of three independent experiments ± S.D., ***, p < 0.001 when compared with control (no H2O2). b, reduced recombinant bGP was incubated with different concentrations of H2O2 (0–250 μm) for 30 min at 37 °C. Residual activity was assayed, and aliquots were also subjected to Western blotting analysis under non-reducing conditions. bGP was revealed using specific antibodies. Results are expressed as the percentage of the control. Data represent mean values of three independent experiments ± S.D., ***, p < 0.001 when compared with positive control. c, reduced recombinant bGP was incubated with glucose oxidase (1.2 units) and glucose (5 mm) for 30 min. In these conditions, H2O2 was continuously generated at a rate of 6 μm/min. An aliquot was removed every 5 min and assayed for bGP residual activity (filled circles). A control reaction was carried out in the presence of 300 units/ml catalase (open circles). Glucose, glucose oxidase, and catalase, independently, had no effect on bGP activity. Samples were analyzed by Western blotting, and bGP was revealed using specific antibodies. Results are expressed as the percentage of the control. Data represent mean values of three independent experiments ± S.D., ***, p < 0.001 when compared with t0. d, to confirm the oxidation of cysteine residues upon exposure to H2O2, recombinant bGP was inhibited by H2O2, and free cysteine residues were specifically labeled using the fluorescent probe 5-IAF. Samples were run on SDS-PAGE in the presence of 2-mercaptoethanol and blotted onto nitrocellulose membrane. 5-IAF fluorescence was measured (λex: 492 nm; λem: 520 nm), and bGP was revealed using specific antibodies. The untreated bGP was used as a positive control. To assess the number of oxidized cysteines after exposure to H2O2, thiol content was analyzed using the DTNB assay on both treated and untreated bGPs, as described under “Experimental Procedures.” The resulting TNB− formation was quantified by absorbance measurement at 405 nm. Absorbance of the non-treated protein was used as control. Data represent mean values of three independent experiments ± S.D., **, p < 0.01 when compared with reduced control. e, recombinant bGP was incubated with various concentrations of H2O2 (0–400 μm H2O2). Aliquots were removed every 5 min and assayed for residual activity. For each concentration of H2O2, the plot of the natural logarithm as a function of time allowed the determination of first-order apparent constants kobs. f, the second-order rate constant was then determined by plotting the first-order apparent constants against [H2O2] (lower panel). The solid lines represent the best linear regression fit of the data to Equations 2 and 3. The calculated kinact for the inhibition of bGP by H2O2 was 185 m−1 min−1. Data represent mean values of three independent experiments ± S.D.