FIGURE 8.
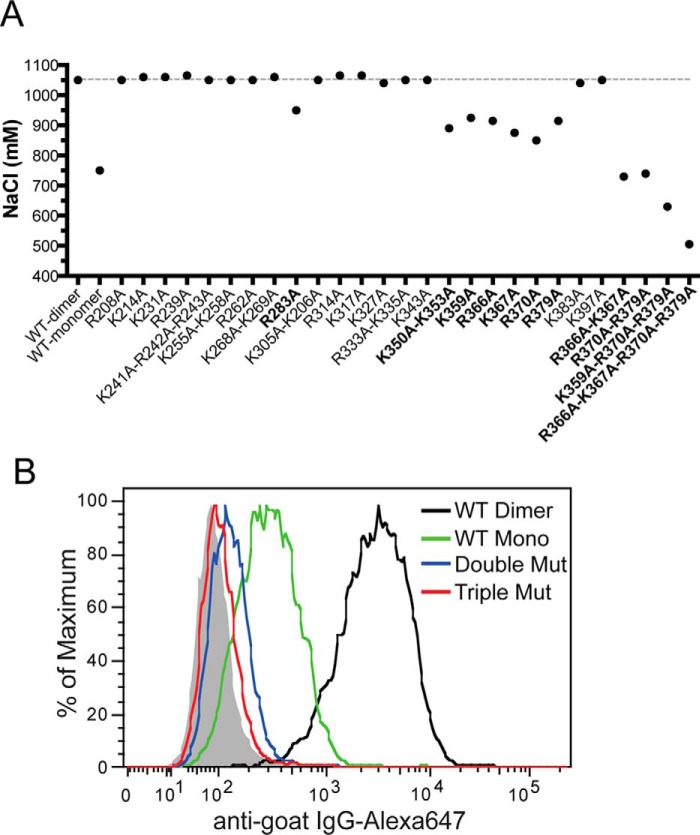
The HS-binding site consists of residues from D2 and Tail domains. A, binding of purified WT or mutants (all dimers) to heparin was analyzed by a 1-ml HiTrap heparin-Sepharose column at pH 7.1. The salt concentrations required for elution are plotted. Mutants that show >100 mm reduction in elution salt concentration are marked in bold. B, binding of OPG mutants (R370A/R379A or K359A/R370A/R379A, all dimers, 33 ng/ml) and wild-type mOPG (dimer and monomer, 33 ng/ml) to cell surface HS was determined by FACS.