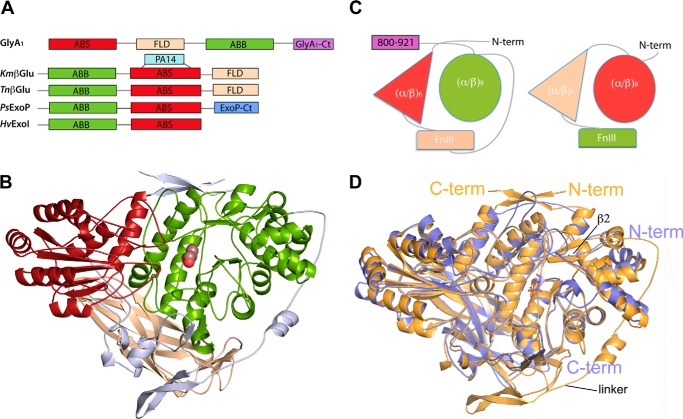
FIGURE 2.
Permuted domain composition of GlyA1. A, comparison of GlyA1 structure with representative members of multidomain GH3 enzymes. β-glucosidases from K. marxianus, KmβGlu (9) and T. neapolitana, TnβGlu (8), the exo-1,3/1,4-β-glucanase from Pseudoalteromonas sp., PsExoP (10) and the barley β-d-glucan exohydrolase, HvExoI (7) are shown. Domains are named as ABS: (α/β)6-sandwich; FLD fibronectin-like; ABB (α/β)8 barrel; PA14, protective antigen PA14 domain. B, folding of GlyA1. The N-terminal (α/β)6-sandwich domain (red) is followed by the FnIII domain (beige) and the (α/β)8 barrel domain (green). Two long segments connect the three domains (gray). A glucose found in the active site is represented by spheres. C, scheme of the GlyA1 domain organization (left) as compared with that of T. neapolitana β-glucosidase (right) (8). D, superimposition of GlyA1 (gold) onto T. neapolitana β-glucosidase (blue) coordinates. Both enzymes present a deviation from the canonical (α/β)8 barrel topology, with their first α-helix missing, which makes strand β2 reversed and antiparallel with the other seven strands. The main difference between both enzymes is the long arm linking the FnIII to the (α/β)8 domain in GlyA1, which is missing in T. neapolitana β-glucosidase. Also, small differences in the orientation of some helixes are observed.