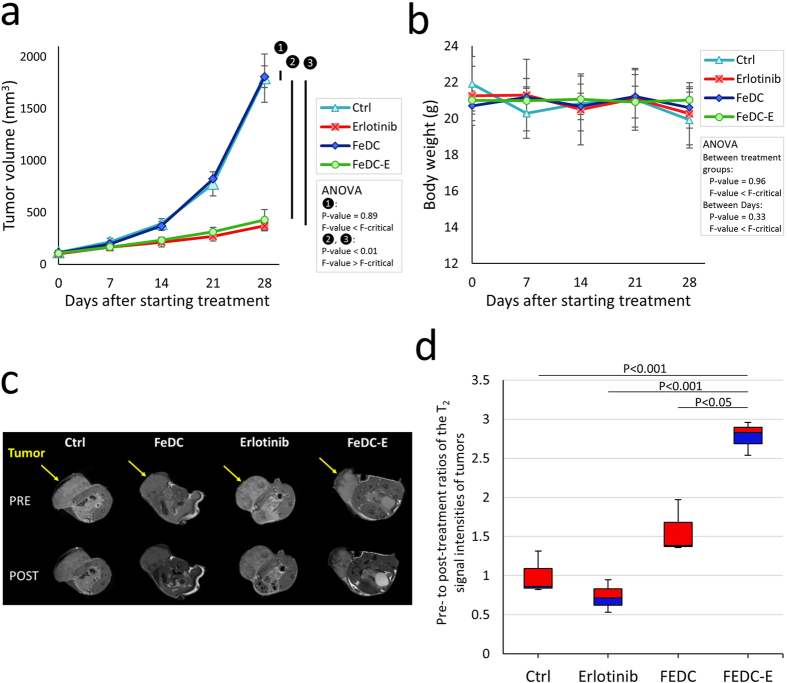
Figure 7. In vivo validation of the antitumor, MRI signal enhancement, and targeting capabilities of the nanoparticles using male BALB/c nude mice bearing xenograft implants of CL1-5-F4 cells.
(a) Tumor volumes of the non-treated mice (Ctrl), erlotinib-treated mice, FeDC NPs-treated mice, and FeDC-E NPs-treated mice through the course of treatment, showing the significant antitumor activities of FeDC-E NPs and erlotinib compared to the control groups. The tumor dimensions were measured using a digital caliper. Please refer to text for the treatment regimen. (b) Body weights of the treated mice throughout the experiment period, showing no indication of toxicity to any of the treatment groups. (c) Representative T2-weighted MRI images of the tested mice acquired pre- and post-treatment for the four groups of mice (arrows point to the tumors). Significant decrease in the MRI signal intensities within tumors of the FeDC-E NPs-treated mice compared to the other treatment groups was observed following initial treatment. (d) Change in the T2-weighted MRI signal intensities within tumors following treatment, presented as ratios of the pre-treatment: post-treatment normalized MRI signal intensities. The FeDC-E NPs-treated mice showed significant drop in the MRI signal intensities within tumors compared to the control non-targeted FeDC NPs-treated group, indicating enhanced targeted accumulation of FeDC-E NPs in the tumors. The P-values for (d) were calculated using the t-Test method assuming unequal variances.