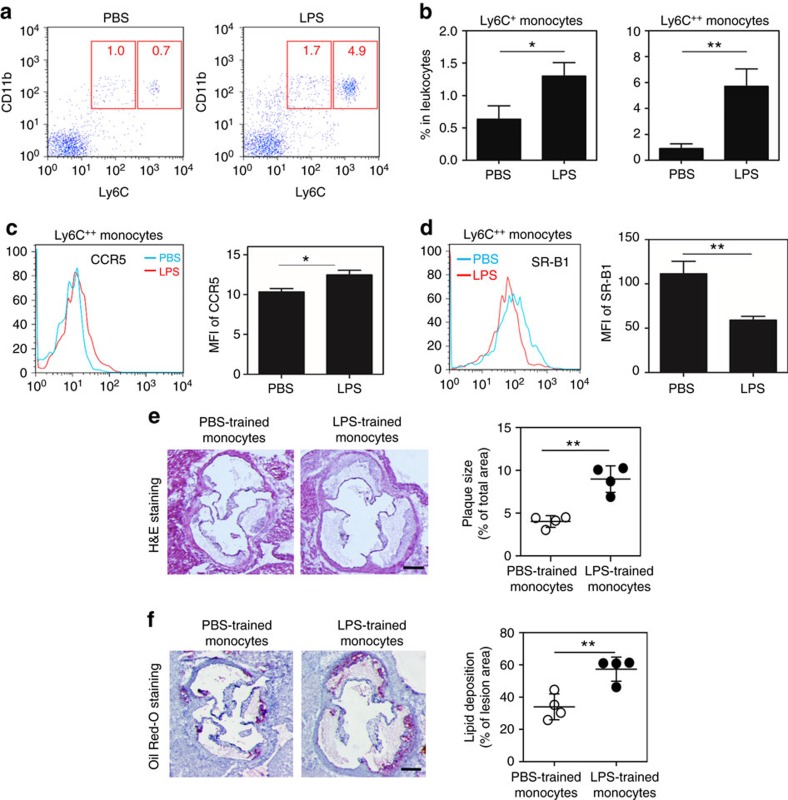
Figure 3. Polarized inflammatory monocytes by subclinical endotoxin contributes to aggravated atherosclerosis.
ApoE−/− mice were pre-conditioned with PBS or super-low-dose LPS for 4 weeks together with HFD, followed by HFD feeding only for an additional 4 weeks. (a) Peripheral blood cells were collected, and CD11b+Ly6C++ and CD11b+Ly6C+ monocytes gated within the Ly6G− population were examined by flow cytometry. (b) The frequency of inflammatory monocytes within total leukocytes was quantified. Data are shown from PBS (n=7) and super-low-dose LPS-conditioned (n=7) mice. (c) The expression levels of CCR5 within circulating inflammatory monocytes were compared between PBS (n=6) and LPS-conditioned (n=6) mice. (d) The expression levels of SR-B1 within circulating inflammatory monocytes were compared between PBS (n=6) and LPS conditioned (n=6) mice. (e,f) Adoptive transfer of LPS-programmed monocytes exacerbates atherosclerosis. BM cells from ApoE−/− mice were cultured with M-CSF (10 ng ml−1) in the presence of PBS or LPS (100 pg ml−1) for 5 days. PBS- or LPS-programmed BM cells (3 × 106 cells per mouse) were then adoptively transferred through intravenous injection to HFD-fed ApoE−/− mice once a week for 4 weeks. (e) Representative images of atherosclerotic plaques within aortic root areas stained by haematoxylin and eosin. Plaque sizes were quantitated as a percentage of the lesion areas within aortic root areas. Scale bar, 300 μm. (f) Representative images of atherosclerotic plaques within aortic root areas stained by Oil Red O. Scale bar, 300 μm. The lipid deposition areas as a percentage of the total atherosclerotic plaques within the aortic root areas were quantified. Data are presentative of two similar experiments. Error bars represent means±s.e.m.; *P<0.05 and **P<0.01; Student's t-test.